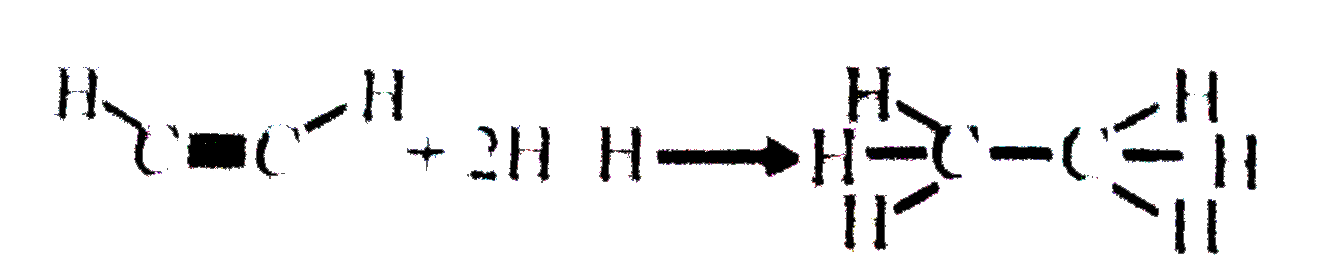A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ALLEN-CHEMISTRY AT A GLANCE-ORGANIC CHEMISTRY
- From the following bond energies :- H-H "bond energy" = 420 KJmol^(-...
Text Solution
|
- IUPAC Name of following compound is :
Text Solution
|
- IUPAC Name for following compound CH(2)=CH-CH(2)-underset(OH)unders...
Text Solution
|
- IUPAC Name for given comp:-
Text Solution
|
- IOPAC name for given compound is
Text Solution
|
- IUPAC Name of :
Text Solution
|
- IUPAC Name of
Text Solution
|
- Which of the following is incorrectly matched :
Text Solution
|
- IUPAC name of (C Cl(3))(3)C Cl is
Text Solution
|
- IUPAC Name of
Text Solution
|
- IUPAC Name of C(6)H(5)COC(2)H(5)
Text Solution
|
- Incorrectly matched with their common names
Text Solution
|
- IUPAC Name for : (CH(3))(2)C(C(2)H(5))(2)
Text Solution
|
- IUPAC Name of : CH(2)=CH-underset(CH(2))underset(||)(C)-C-=CH
Text Solution
|
- IUPAC Name of the given compound CH(3)-underset(O)underset(||)(C)-C...
Text Solution
|
- IUPAC Name for given compound : CH(3)-underset(CH(3))underset(|)(CH...
Text Solution
|
- IUPAC name of the following compound is CH(3)-CH=CH-CH(2)-C-=CH
Text Solution
|
- IUPAC name of given compound is
Text Solution
|
- Which of the following IUPAC name is incorrect
Text Solution
|
- Number of carbon in parent carbon chain in the following compound
Text Solution
|
- The correct IUPAC name of following compound is :
Text Solution
|