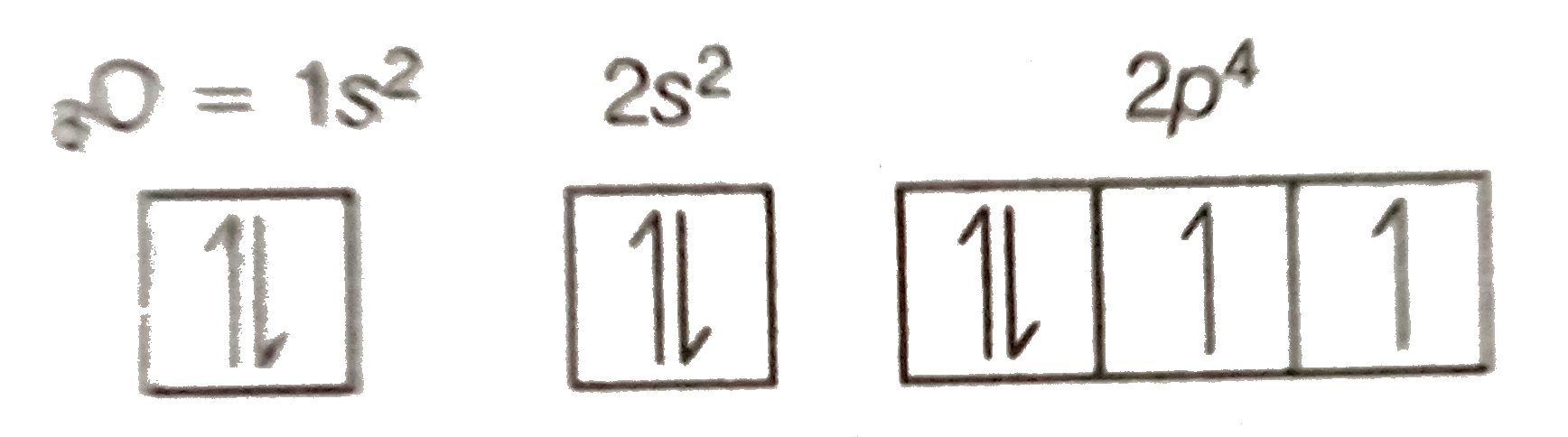Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NCERT EXEMPLAR-STRUCTURE OF ATOM-All Questions
- Which of the following statements concerning the quantum numbers are c...
Text Solution
|
- Arrange s,p and d subshells of a shell in the increasing order of effe...
Text Solution
|
- Show the distribution of electrons in oxygen atom (atomic number 8) us...
Text Solution
|
- Nickel atom can lose two electrons to form Ni^(2+) ion. The atomic num...
Text Solution
|
- Which of the following orbitals are degernate? 3d(xy),4d(xy),3d(z^(...
Text Solution
|
- Calculate the total number of angular nodes and radical nodes present ...
Text Solution
|
- The arrangement of orbitals on the basis of energy is based upon their...
Text Solution
|
- Which of the following will not show deflection from the path on passi...
Text Solution
|
- An atom having atomic mass number 13 has 7 neutrons. What is the atomi...
Text Solution
|
- Wavelength of different ra diations are given below. lambda (A) = 3...
Text Solution
|
- The electronic configuration of valence shell of Cu is 3d^(10)4s^(1) a...
Text Solution
|
- The Balmer series in the hydrogen spectrum corresponds to the transiti...
Text Solution
|
- According to de-Brogile, matter should exhibit dual behaviour, that i...
Text Solution
|
- What is the experimental evidence in support of the diea that electron...
Text Solution
|
- Out of electron and proton which one will have, a higher velocity to p...
Text Solution
|
- A hypothetical electromagnetic wave is shown in figure. Find out the w...
Text Solution
|
- Chlorophyll present in green leaves of plants absorbs light at 4.620xx...
Text Solution
|
- What is the difference between the terms orbit and orbital?
Text Solution
|
- Table-tennis ball has mass 10g and s peed of 90m/s. if speed can be me...
Text Solution
|
- The effect of uncertainty principle is significant only for motion of ...
Text Solution
|