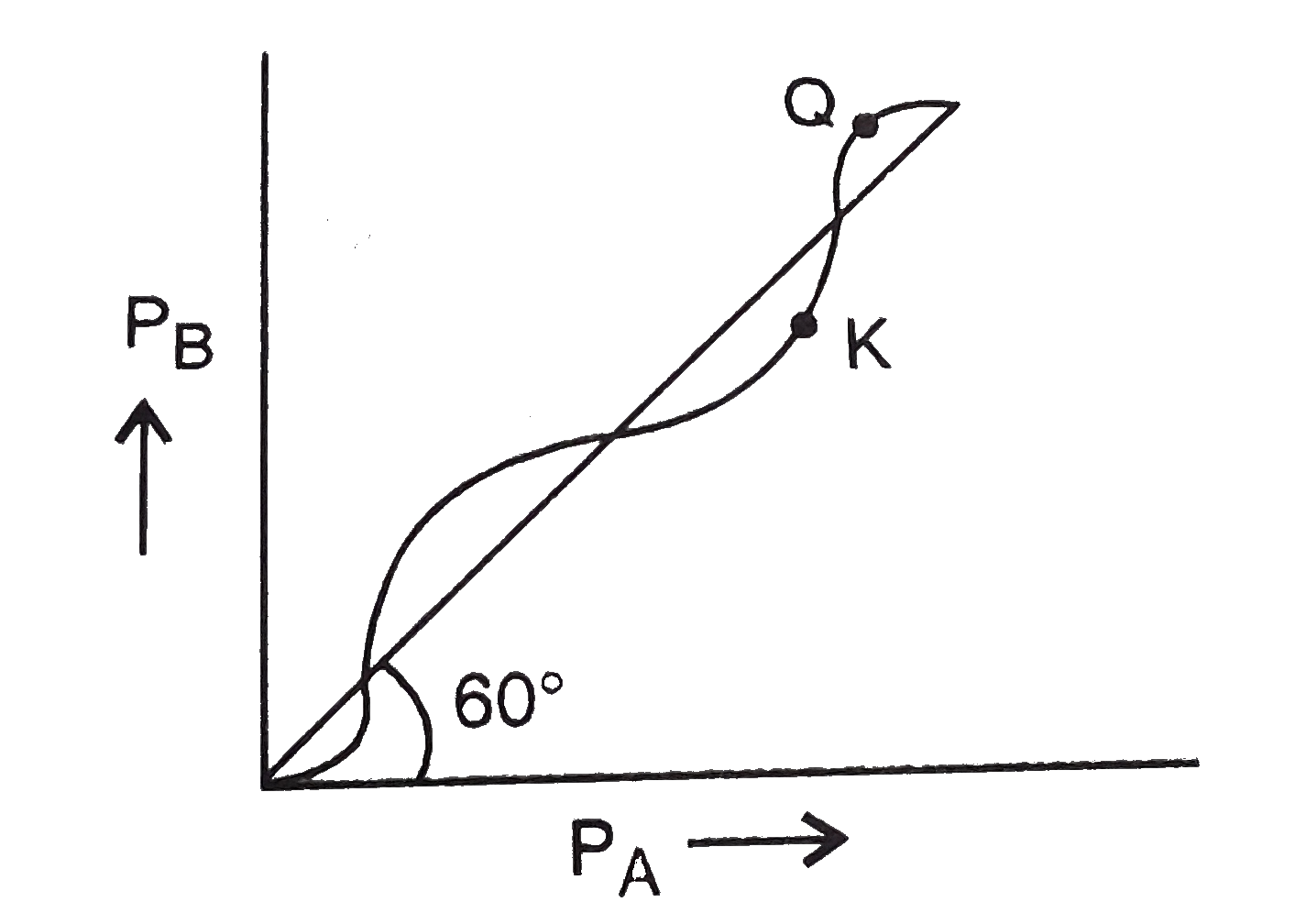
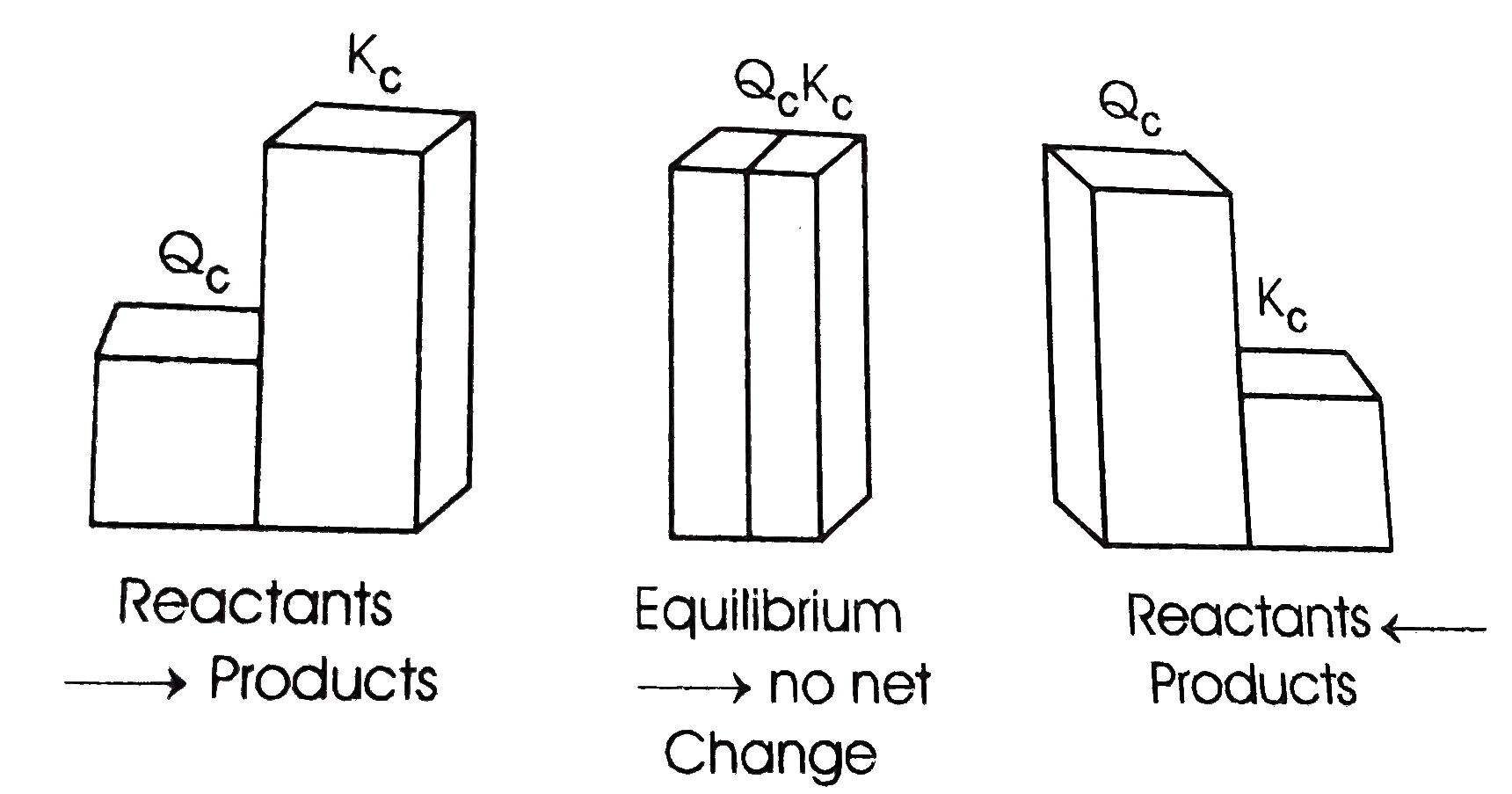A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
PHYSICAL AND CHEMICAL EQUILIBRIA
DINESH PUBLICATION|Exercise MATRIX MATCH TYPE MCQs|2 VideosPHYSICAL AND CHEMICAL EQUILIBRIA
DINESH PUBLICATION|Exercise REASON ASSERTION TYPE MCQs|10 VideosPHYSICAL AND CHEMICAL EQUILIBRIA
DINESH PUBLICATION|Exercise MCQs with only one correct answer|24 VideosP BLOCK ELEMENTS (GROUP 13 AND 14 )
DINESH PUBLICATION|Exercise Straight obj.|17 VideosRATES OF REACTIONS AND CHEMICAL KINETICS
DINESH PUBLICATION|Exercise Ultimate Preparatory Package|29 Videos
Similar Questions
Explore conceptually related problems
DINESH PUBLICATION-PHYSICAL AND CHEMICAL EQUILIBRIA-LINKED COMPREHENSION TYPE MCQ s (Comprehension 1)
- The reaction which is in dynamic equilibrium, ensured us, that the rea...
Text Solution
|
- The reaction which is in dynamic equilibrium, ensured us, that the rea...
Text Solution
|
- The reaction which is in dynamic equilibrium, ensured us, that the rea...
Text Solution
|
- The reaction which is in dynamic equilibrium, ensured us, that the rea...
Text Solution
|
- The reaction which is in dynamic equilibrium, ensured us, that the rea...
Text Solution
|
- The reaction which is in dynamic equilibrium, ensured us, that the rea...
Text Solution
|
- The reaction which is in dynamic equilibrium, ensured us, that the rea...
Text Solution
|
- The reaction which is in dynamic equilibrium, ensured us, that the rea...
Text Solution
|