A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NCERT EXEMPLAR-THE P-BLOCK ELEMENTS-Long Answer
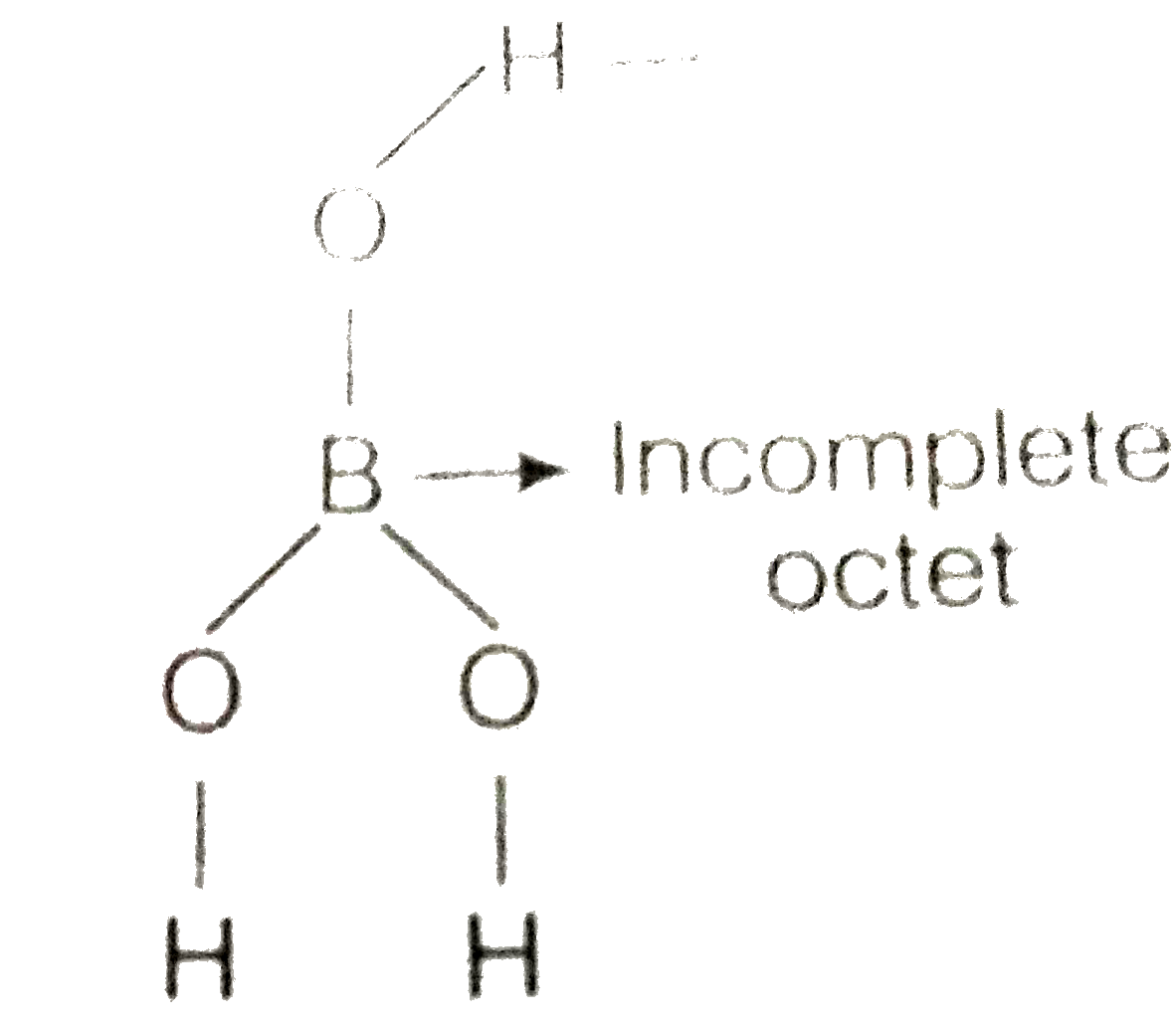
- Boric acid is an acid because its molecule
Text Solution
|
- Describe the general trends in the following properties of the element...
Text Solution
|
- Account for the following observations (a) AlCl(3) is a Lewis acid ...
Text Solution
|
- When aqueous solution of borax is acidified with hydrochloric acid, a ...
Text Solution
|
- Three pairs of compounds are given below, identify that compound in e...
Text Solution
|
- BCl(3) exists as monomer whereas AlCl(3) is dimerised through halogen ...
Text Solution
|
- Boron fluoride exists as BF(3) but boron hydride does't exist as BH(3)...
Text Solution
|
- (a) What are silicones ? States the uses of silicones (b) What are b...
Text Solution
|
- A compound (A) of boron reacts with Nme(3) to give an adduct (B) which...
Text Solution
|
- A non-metallic element of group 13, used in making bullet prrof vests ...
Text Solution
|
- A tetravalent element forms monoxide and dioxide with oxygen. When air...
Text Solution
|
 .
.