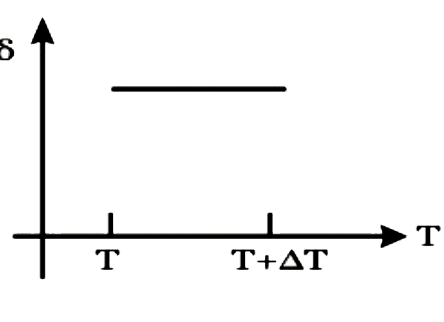
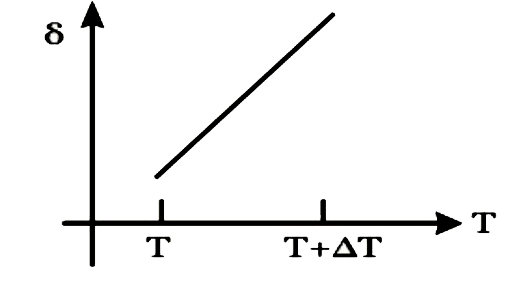
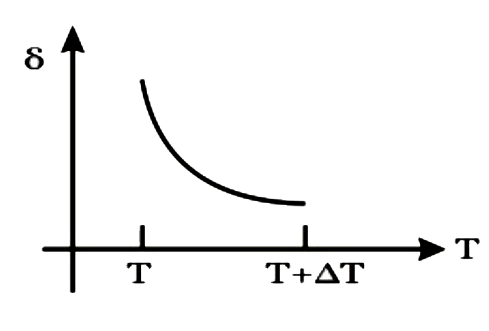
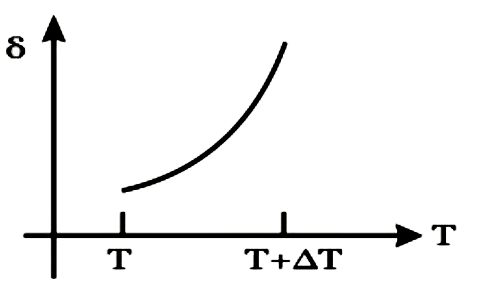
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NTA MOCK TESTS-NTA NEET SET 53-PHYSICS
- The temperature of sun is 5500 K and it emits maximum intensity radiat...
Text Solution
|
- The variation of photocurrent with collector potential for different f...
Text Solution
|
- An ideal gas is initially at temperature T and volume V . Its volume i...
Text Solution
|
- A gas can be taken from A to B via two different processes ACB and DB...
Text Solution
|
- A truck of mass 10 metric ton runs at 3ms^(-1) along a level track and...
Text Solution
|
- A thin flexible wire of length L is connected to two adjacent fixed po...
Text Solution
|
- A one mole of an ideal gas expands adiabatically at constant pressure...
Text Solution
|
- A cylindrical conductor of diameter 0.1 mm carries a current of 90 ma....
Text Solution
|
- In an AC circuit , current is 3A and voltage 210V and power is 63W. Th...
Text Solution
|
- The upper half of an inclined plane with inclination phi is perfectly ...
Text Solution
|
- The potential energy for a conservative force system is given by U=ax^...
Text Solution
|
- Starting with a sample of pure .^(66)Cu, 7//8 of it decays into Zn in ...
Text Solution
|
- The ends of a rod of length l and mass m are attached to two identica...
Text Solution
|
- A pendulum is executing simple harmonic motion and its maximum kinet...
Text Solution
|
- A photocell is illuminated by a small bright source places 1 m away w...
Text Solution
|
- The magnitude of x and y components of vec(A) are 7 and 6 respectively...
Text Solution
|
- The work done in blowing a bubble of volume V is W, then what is the w...
Text Solution
|
- A body is projected at time t = 0 from a certain point on a planet's s...
Text Solution
|
- A body of mass 8 kg is suspended through two light springs X and Y con...
Text Solution
|
- The two surfaces of a biconvex lens has same radii of curvatures . Thi...
Text Solution
|