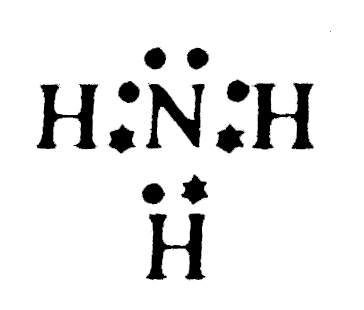A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
DINESH PUBLICATION-CHEMICAL BONDING AND MOLECULAR STRUCTURE-Unit Test - 1
- In multiplication and division the significant figures of answer must ...
Text Solution
|
- How many significant figures are there in (respectively) (1) 73.000 ...
Text Solution
|
- The sample with largest number of atoms is
Text Solution
|
- Number of atoms in 560gm of Fe(atomic mass 56gm " mol"^(-1)) is:
Text Solution
|
- About a gaseous reaction xX+yY to l L +mM which statement is wron...
Text Solution
|
- Pick out the isoelectronic structures from the following underset (I...
Text Solution
|
- The radius of which of the following orbit is same as that of the firs...
Text Solution
|
- Which of the following sets of quantum numbers represents an impo...
Text Solution
|
- The electrons present in K-shell of the atom will differ in
Text Solution
|
- The increasing order (lowest first) for the values of e//m (charge//ma...
Text Solution
|
- The incorrect statement Among the following is A)The first ionisation...
Text Solution
|
- The screening effect of inner electrons of an atom can cause
Text Solution
|
- Which of the following transitions involves maximum amount of energy?
Text Solution
|
- The set representing the correct order of the first ionisation potenti...
Text Solution
|
- Which one of the following elements has the highest ionisation energy?
Text Solution
|
- Number of electrons in the valence orbit of nitrogen in an ammonia mol...
Text Solution
|
- The dipole moment of HBr is 1.6 xx 10^(-30) cm and interatomic spaci...
Text Solution
|
- When two atoms of chlorine combine to form one molecule of chlorine ga...
Text Solution
|
- In which of the following the central atom does not use sp^3 hybrid or...
Text Solution
|
- What is the effect of more electronegative atoms on the strength of an...
Text Solution
|