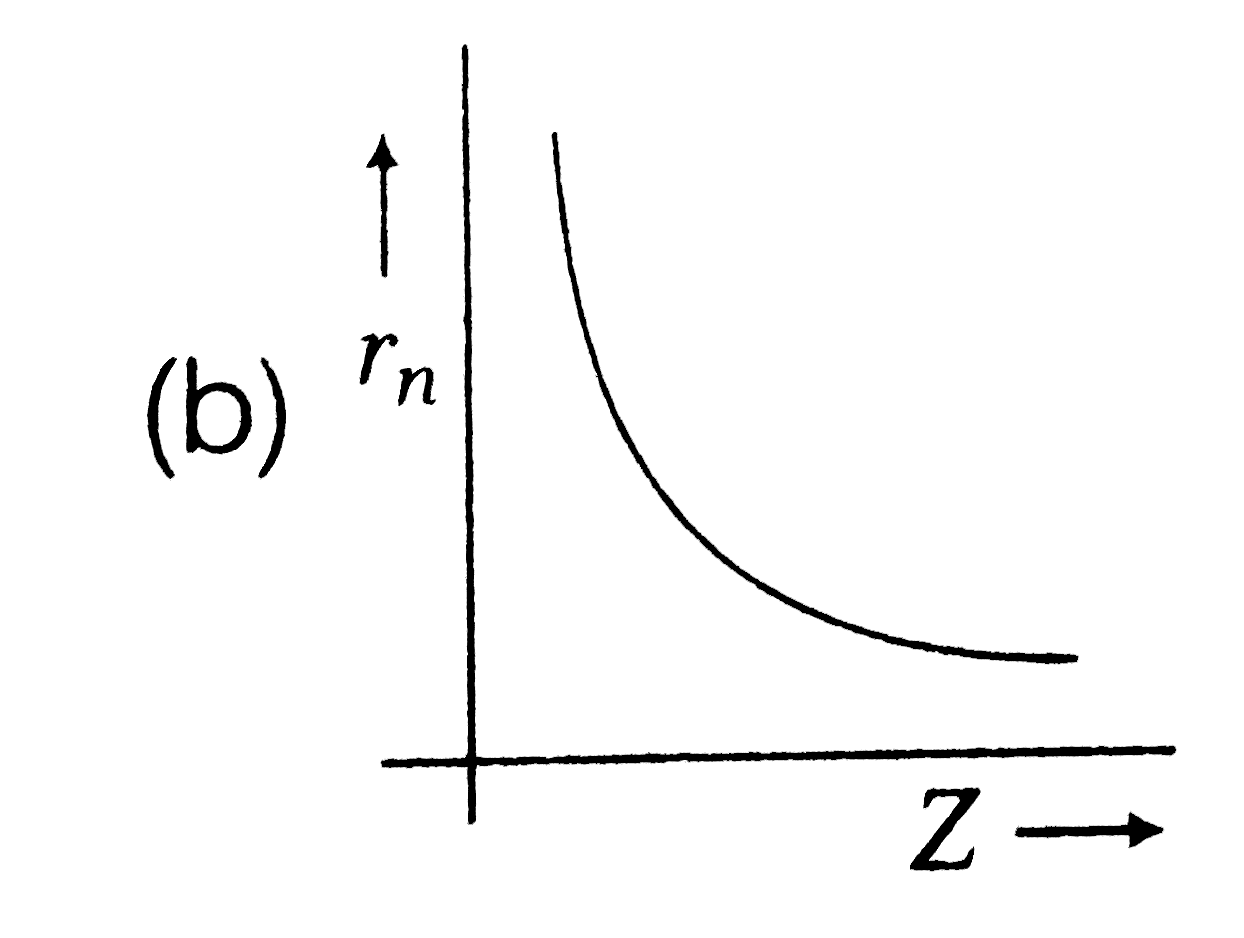
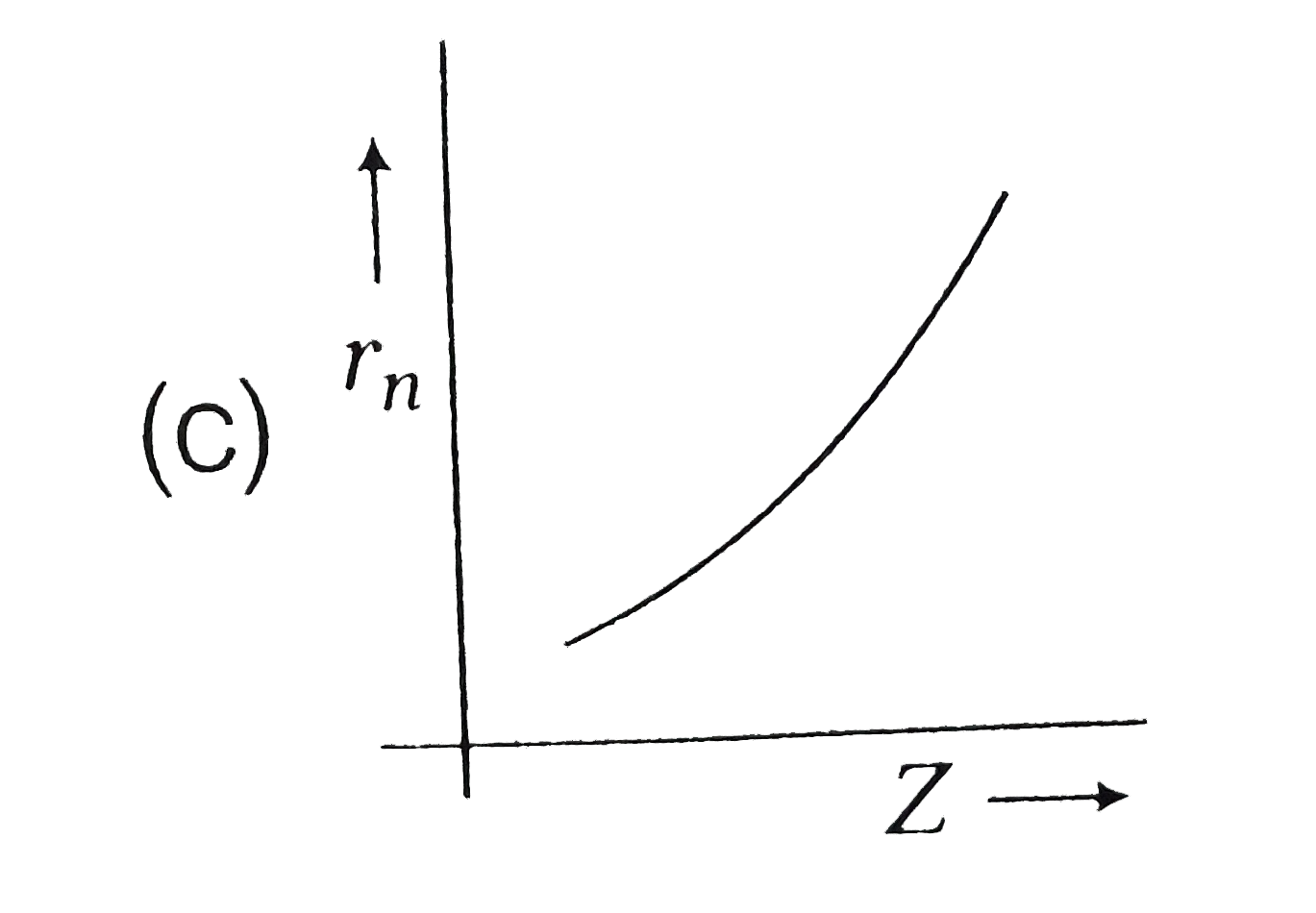
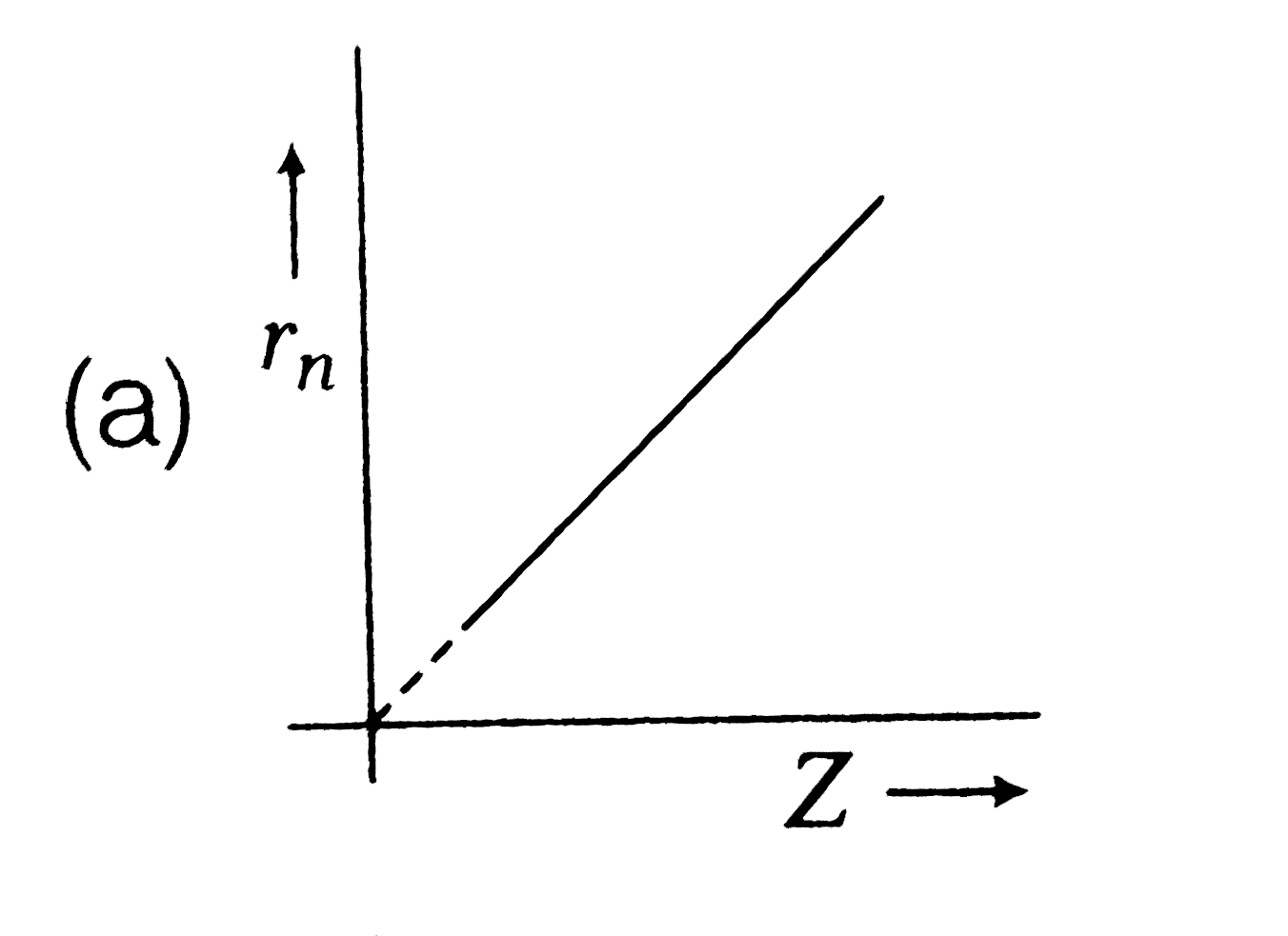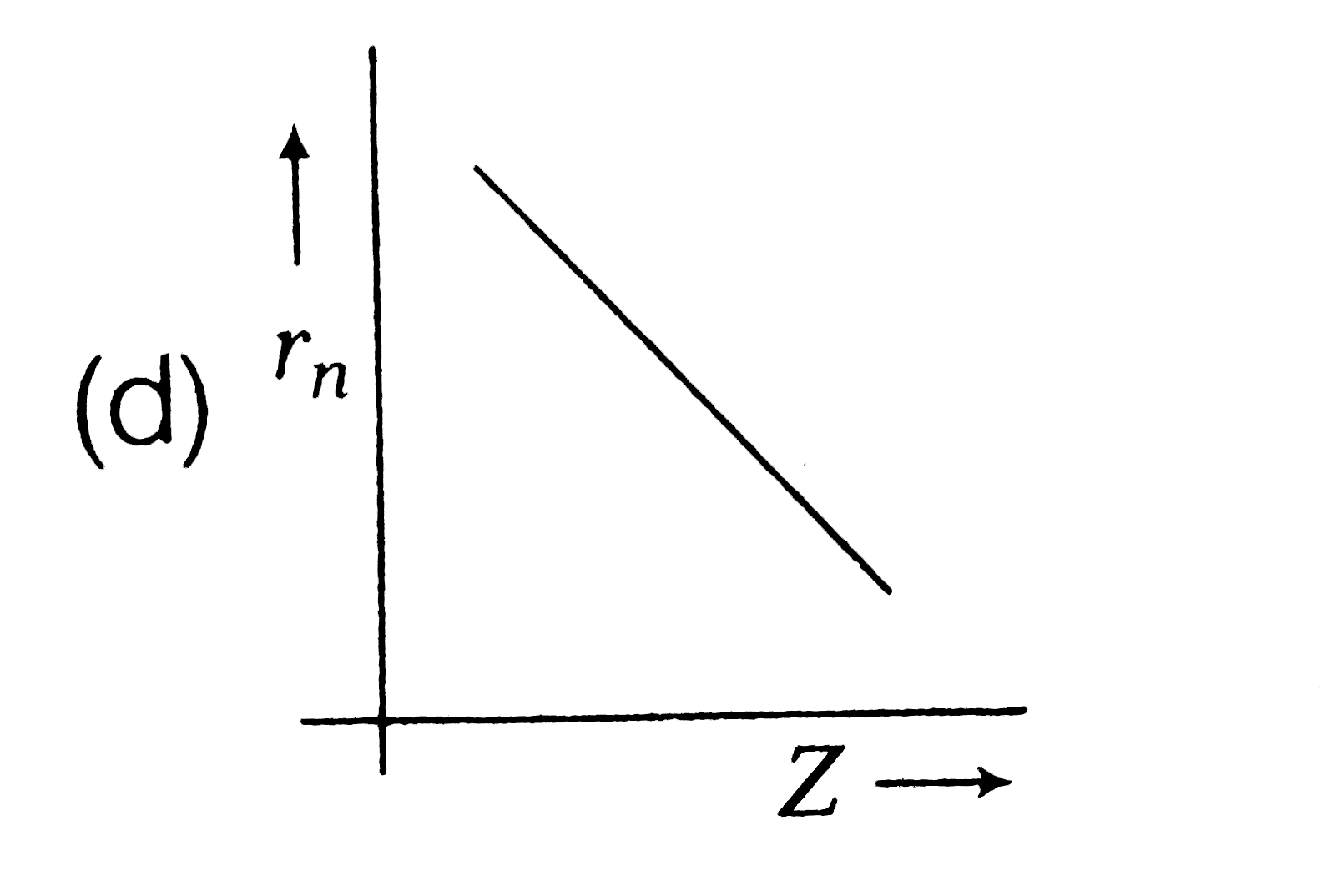A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
DC PANDEY-ATOMS-Check point 12.1
- The radius of the atom is of the order of
Text Solution
|
- According to classical theory, the circular path of an electron in Rut...
Text Solution
|
- An alpha nucleus of energy (1)/(2)m nu^(2) bombards a heavy nucleus o...
Text Solution
|
- The number of alpha-particless scattered per unit area N (theta) at sc...
Text Solution
|
- in Rutherford scattering experiment for scattering angle of 180^(@) , ...
Text Solution
|
- The concept of stationary orbits was proposed by
Text Solution
|
- In Bohr's atom model,
Text Solution
|
- The angular momentum (L) of an electron moving in a stable orbit aroun...
Text Solution
|
- In Bohr's mode, the atomic radius of the first orbit is r(0) then the ...
Text Solution
|
- In which of the following systems will the radius of the first orbit (...
Text Solution
|
- Radius (r(n)) of electron in nth orbit versus atomic number (Z) graph ...
Text Solution
|
- The ratio of the speed of the electrons in the ground state of hydroge...
Text Solution
|
- Speed (V(n)) of electron in nth orbit versus principal quantum number ...
Text Solution
|
- The orbital frequency of an electron in the hydrogen atom is proportio...
Text Solution
|