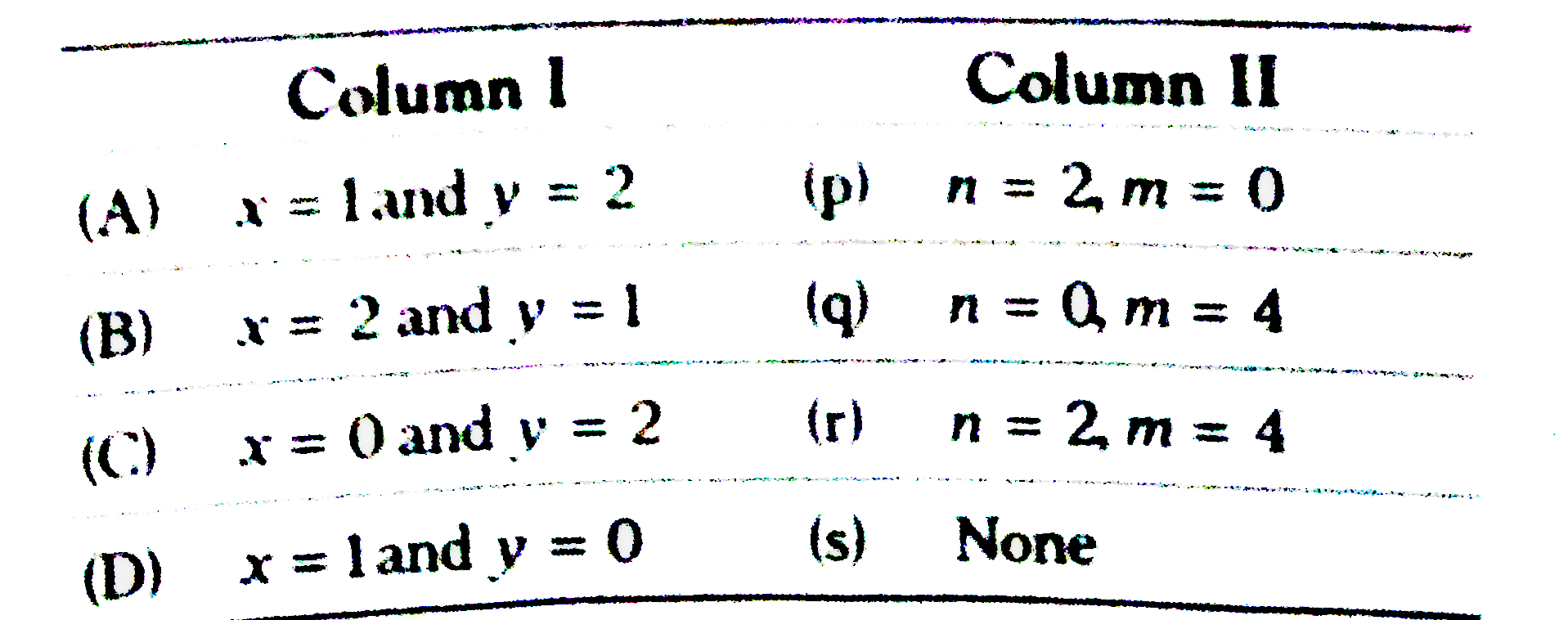Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
DC PANDEY-NUCLEI-MATCH THE COLUMNS
- From a radioactive substance x numbers of alpha-particles any y number...
Text Solution
|
- Match the statement in Column I to the approprate statement(s) from Co...
Text Solution
|
- Match the statement in Column I to the appropriate statement(s) from C...
Text Solution
|
- Match the Clumn I of the nuclear processes with Column II containing p...
Text Solution
|