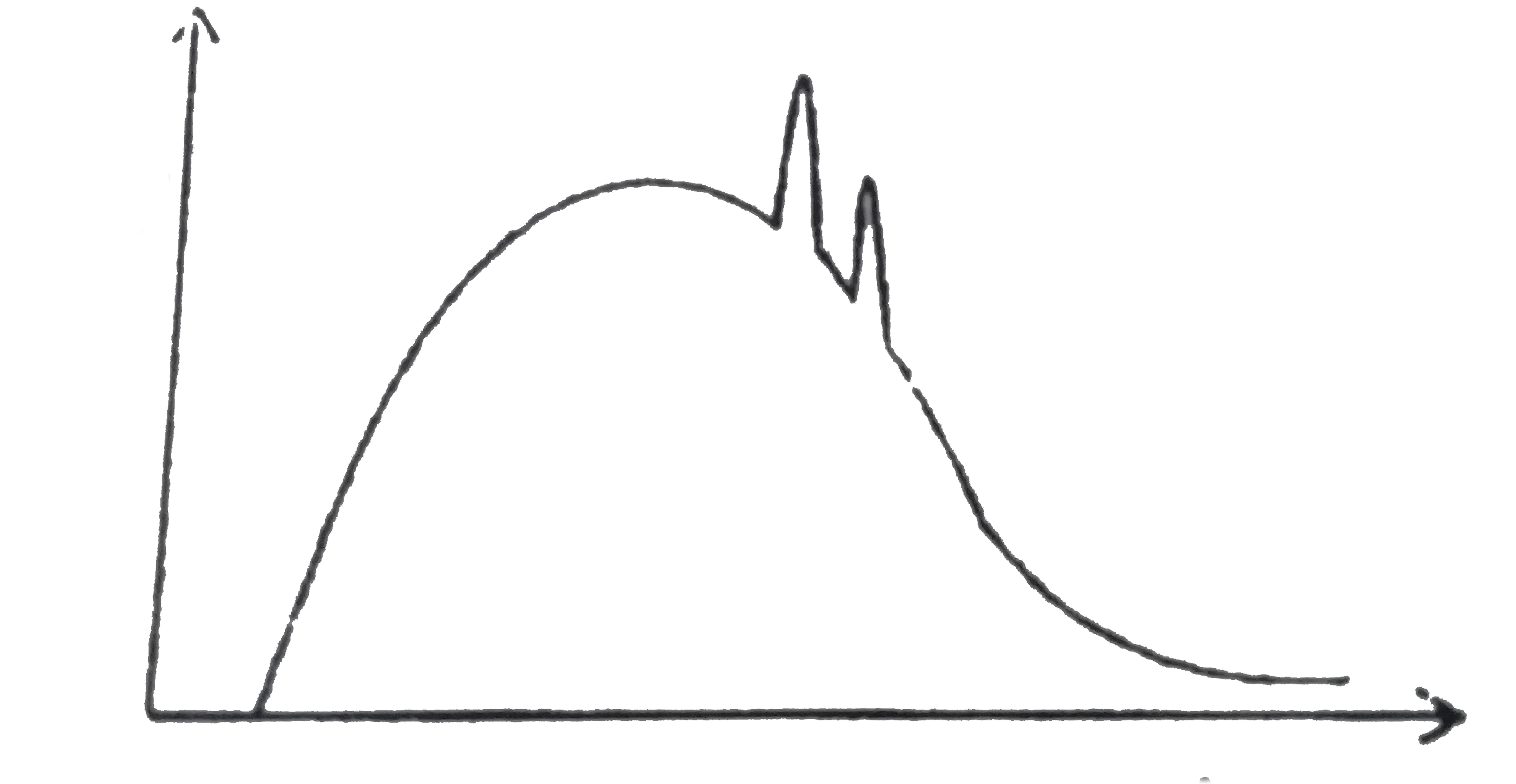
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NTA MOCK TESTS-NTA JEE MOCK TEST 86-PHYSICS
- A beam of electrons striking a cooper target produces X-rays. Its spec...
Text Solution
|
- A ball falls freely from a height of 45m. When the ball is at a height...
Text Solution
|
- A particle of mass m moves along the internal smooth surface of a vert...
Text Solution
|
- Two identical metal plates are given poistive charges Q1 and Q2 (ltQ1)...
Text Solution
|
- A planet moving around the sun sweeps area A(1) in 2 days, A(2) in 4 d...
Text Solution
|
- Two capacitors C(1) and C(2) in a circuit are joined as shown. If V(A)...
Text Solution
|
- A long wire carries a steady curent . It is bent into a circle of one ...
Text Solution
|
- Two seconds after projection, a projectile is travelling in a directio...
Text Solution
|
- A wooden wedge of mass M and inclination anlgle(alpha) rest on a smoot...
Text Solution
|
- If a proton and anti-proton come close to each other and annihilate, h...
Text Solution
|
- In an experiment , to find the loss of energy with respect to time in ...
Text Solution
|
- In an experiment on photoelectric emission from a metallic surface ,w...
Text Solution
|
- The lower end of a glass capillary tube is dipped in water. Water rise...
Text Solution
|
- A small solid sphere of radius r rolls down an incline without slippin...
Text Solution
|
- For CE transistor amplifier, the audio signal voltage across the colle...
Text Solution
|
- A body takes 10 minutes to cool from 60^(@)C to 50^(@)C. The temperatu...
Text Solution
|
- The potential energy of a particle varies with distance x from a fixed...
Text Solution
|
- In a double slit experiment, the two slits are 1 mm apart and the scre...
Text Solution
|
- A train moves towards a stationary observer with speed 34 m//s. The tr...
Text Solution
|
- The potential energy of a particle of mass 5 kg moving in the x-y plan...
Text Solution
|