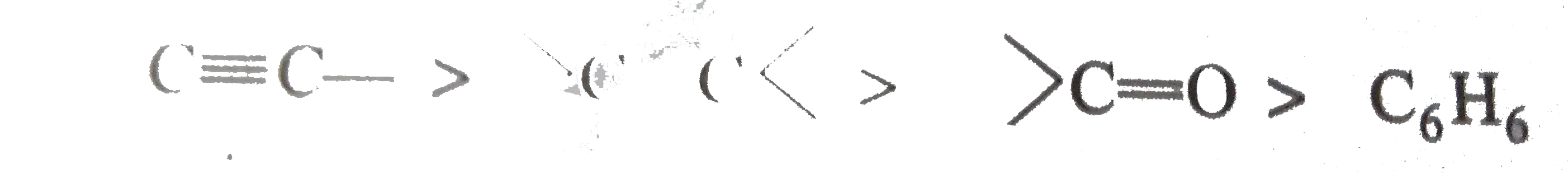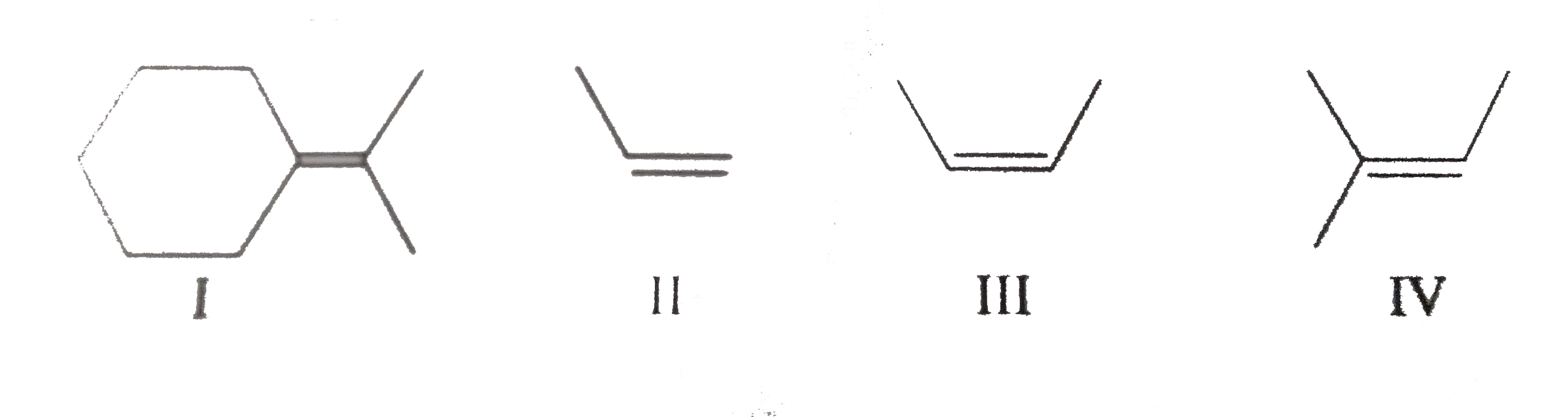A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
OP TANDON-UNSATURATED HYDROCARBONS-LINKED COMPREHENSION TYPE QUESTIONS
- Hydrogenation of alkenes and alkynes takes place in presence of certai...
Text Solution
|
- Hydrogenation of alkenes and alkynes takes place in presence of certai...
Text Solution
|
- Hydrogenation of alkenes and alkynes takes place in presence of certai...
Text Solution
|
- Hydrogenation of alkenes and alkynes takes place in presence of certai...
Text Solution
|
- Hydrogenation of alkenes and alkynes takes place in presence of certai...
Text Solution
|
- Oxidation without clevage of sigma bond takes place in alkenes. P...
Text Solution
|
- Oxidation without clevage of sigma bond takes place in alkenes. P...
Text Solution
|
- Oxidation without clevage of sigma bond takes place in alkenes. P...
Text Solution
|
- Oxidation without clevage of sigma bond takes place in alkenes. P...
Text Solution
|
- Oxidation without clevage of sigma bond takes place in alkenes. P...
Text Solution
|
- Oxidation of alkenes by cleavage with the acidic or alkaline KmNO(4) o...
Text Solution
|
- Oxidation of alkenes by cleavage with the acidic or alkaline KmNO(4) o...
Text Solution
|
- Oxidation of alkenes by cleavage with the acidic or alkaline KmNO(4) o...
Text Solution
|
- Oxidation of alkenes by cleavage with the acidic or alkaline KmNO(4) o...
Text Solution
|
- Oxidation of alkenes by cleavage with the acidic or alkaline KmNO(4) o...
Text Solution
|
- An alkene (A), C(5)H(12) on chlorination at 300^(@)C gives a mixture o...
Text Solution
|
- An alkene (A), C(5)H(12) on chlorination at 300^(@)C gives a mixture o...
Text Solution
|
- An acylic hydrocarbons P, having molecular formula C(6)H(10), gave ace...
Text Solution
|
- An acylic hydrocarbons P, having molecular formula C(6)H(10), gave ace...
Text Solution
|
- In the following reactions Answer the following questions:
Text Solution
|