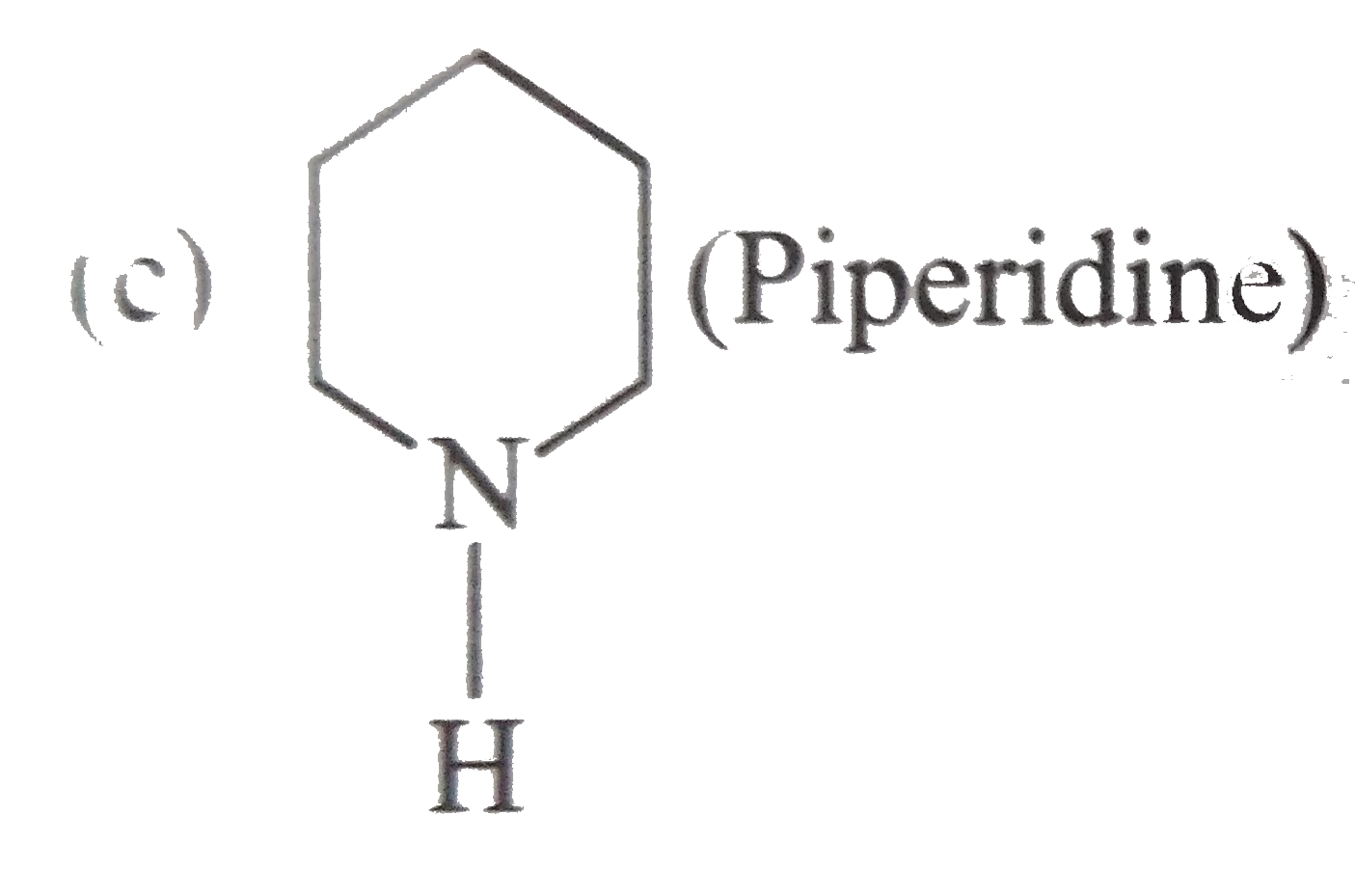
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
ORGANIC COMPOUNDS CONTAINING NITROGEN
OP TANDON|Exercise STEP II:|20 VideosORGANIC COMPOUNDS CONTAINING NITROGEN
OP TANDON|Exercise PASSAGE|16 VideosORGANIC COMPOUNDS CONTAINING NITROGEN
OP TANDON|Exercise Level A|213 VideosISOMERISM (STRUCTURAL AND STEROISOMERISM)
OP TANDON|Exercise INTEGER|7 VideosRADIOACTIVITY AND NUCLEAR TRANSFORMATION
OP TANDON|Exercise SECTION - VI|3 Videos
Similar Questions
Explore conceptually related problems
OP TANDON-ORGANIC COMPOUNDS CONTAINING NITROGEN-STEP I:
- Which nitrogen is protonated readily in the guanidine?
Text Solution
|
- Which of the following has the highest pK(b) value?
Text Solution
|
- Which of the following is insoluble in dil.HCl
Text Solution
|
- Which of the following may be prepared by Galbriel phthalimide synthes...
Text Solution
|
- When an organic compound was treated with sodium nitrite and hydrochlo...
Text Solution
|
- In carbylamine reaction:
Text Solution
|
- Which one of the following will not react with the Grignard reagent (C...
Text Solution
|
- One mole of an amine (A) consumes two moles of methyl bromide to give ...
Text Solution
|
- An optically active compound (A) decolourises Br(2)//C Cl(4) and relea...
Text Solution
|
- (A) overset(H(2)//Pt)to1^(@)"Amine" (B) overset(H(2)//Pt)to2^(@)"Ami...
Text Solution
|
- How many products will be obtained when propane is subjected to vapour...
Text Solution
|
- Arrange following amines in the decresing order of their basicity:
Text Solution
|
- Which of the following reactions is feasible?
Text Solution
|
- Predict the product
Text Solution
|
- In gattermann reaction a diazonium group is replaced by X using Y. Wh...
Text Solution
|
- An orange dye p-hydroxy azobenzene can be synthesized from benzene dia...
Text Solution
|
- Identify the product in the following reaction:
Text Solution
|
- The most basic amine among the following is:
Text Solution
|
- Consider the nitratio of benzene using mixed conc. H(2)SO(4) and HNO(3...
Text Solution
|
- The correct statement regarding the basicity of arylamines is .
Text Solution
|