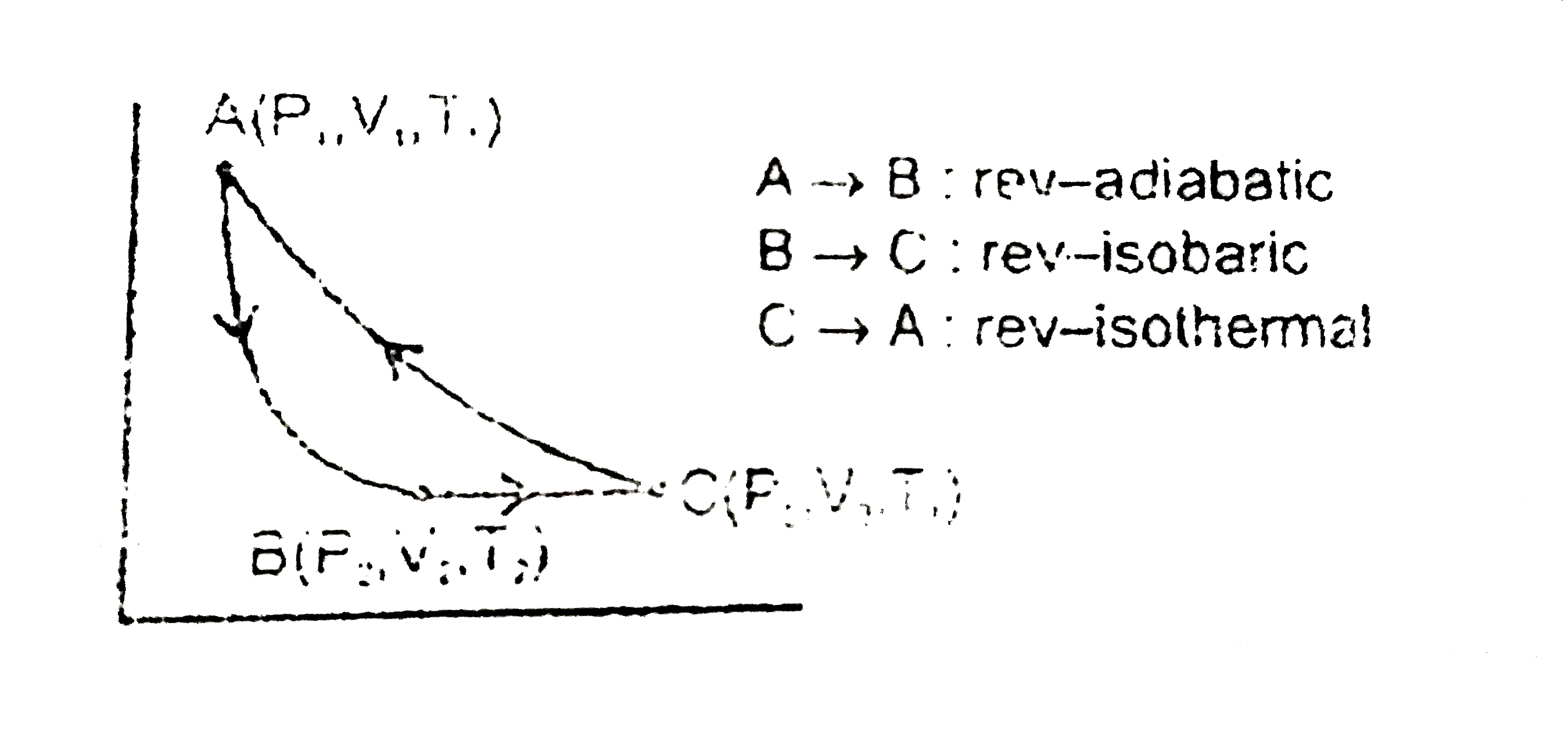Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
THERMODYNAMICS
RESONANCE|Exercise exercise-3 Part-3 :(Subjective questions)Section C|6 VideosTHERMODYNAMICS
RESONANCE|Exercise exercise-3 Part-3 :(Only one option correct type) section A|8 VideosTHERMODYNAMICS
RESONANCE|Exercise exercise-3 Part-3 :(Subjective questions)Section A|1 VideosTEST SERIES
RESONANCE|Exercise CHEMISTRY|50 Videos
Similar Questions
Explore conceptually related problems
RESONANCE-THERMODYNAMICS-exercise-3 Part-3 :(Subjective questions)Section B
- One mole of monoatomic has was taken through a cyclic process as shown...
Text Solution
|
- The entropy if vaporisation of benzene is 85JK^(-1) mol^(-1). When 117...
Text Solution
|
- One mole of ideal monatomic gas was taken through isochoric heating fr...
Text Solution
|
- Calculate standard entropy change in the reaction Fe(2)O(3)(s)+3H(2)...
Text Solution
|
- One mole of solid iron was vaporized in an oven at 3500 K . If iron bo...
Text Solution
|
- Calculate the entropy change in surroundings when 1.00 mol of H(2)O(l)...
Text Solution
|
- Order of increasing of entropy ammong given condition of substance is ...
Text Solution
|
- Oxygen & ozone are gases at standard temperature. Their molar entropie...
Text Solution
|