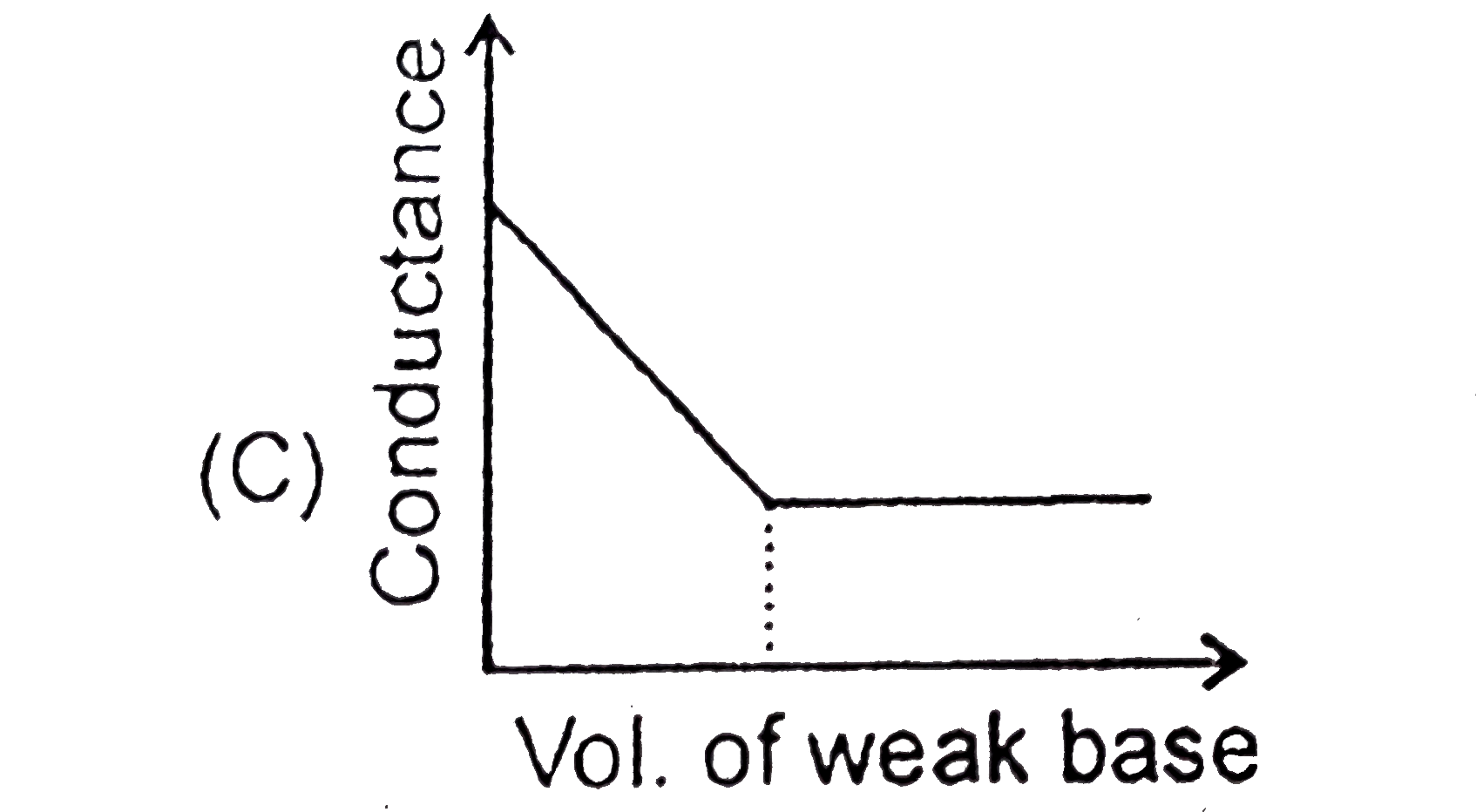
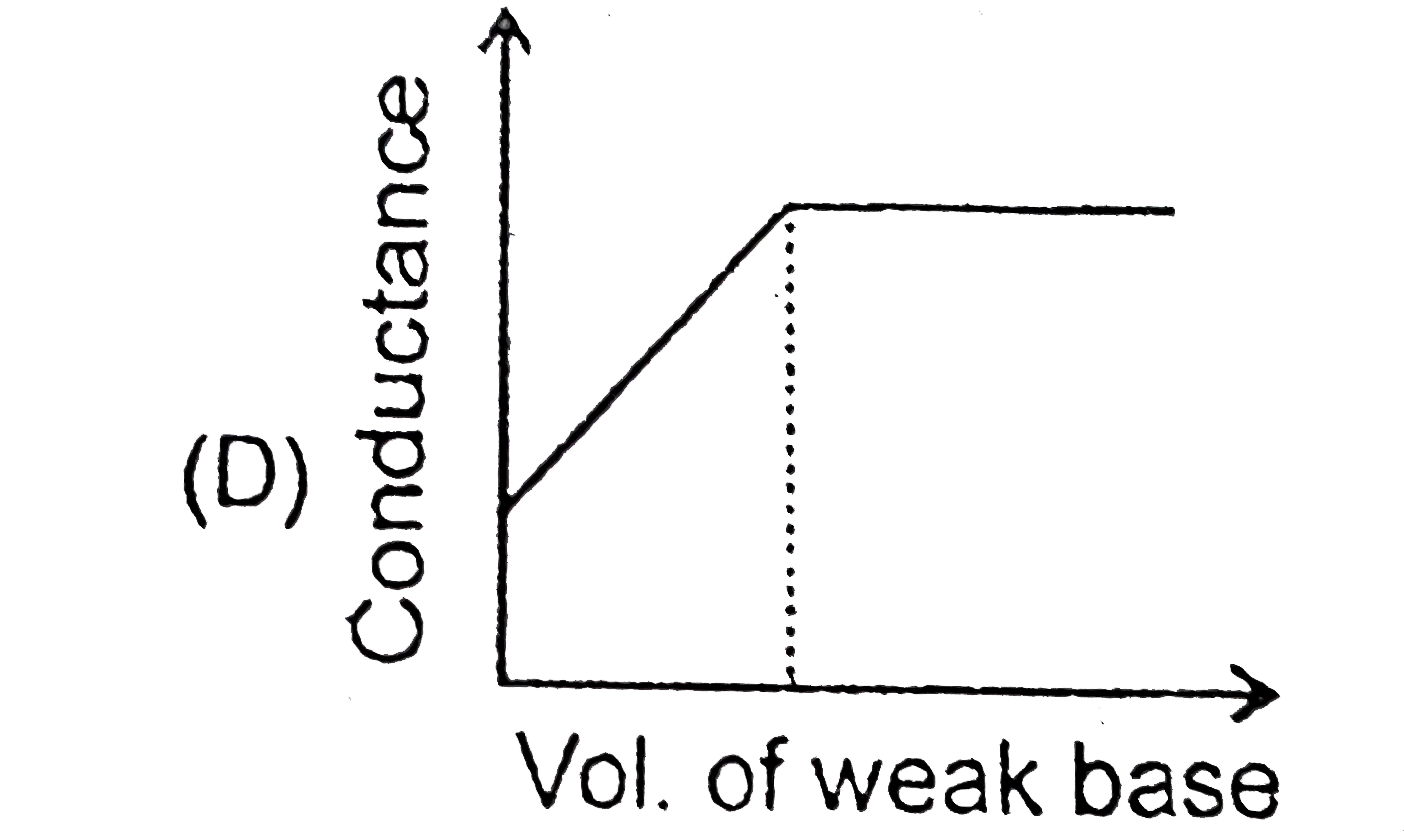
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
RESONANCE-TEST SERIES-CHEMISTRY
- Which of the following plots will obtained for a conductomeric titrati...
Text Solution
|
- Freundlich adsorption isotherm is given by the expression x/m=kP^(1/n)...
Text Solution
|
- The correct figure representing isothermal and adiabatic compression o...
Text Solution
|
- Which of the following reactions does not occur during calcination?
Text Solution
|
- Which of the following compounds can show geometrical optical and conf...
Text Solution
|
- Which of the following is the strongest nucleophile?
Text Solution
|
- Which of the following are low spin complexes which follow EAN rule?
Text Solution
|
- Which of the following statement(s) is/are correct?
Text Solution
|
- ^^(m)^(0)H(2)O is equal to
Text Solution
|
- Which of the following is/are oxide ores?
Text Solution
|
- Zn Amalgan is prepated by electrolysis of aqueous ZnCl(2) using Hg ca...
Text Solution
|
- In which of the following electrolysis in aqueous medium, mass of anod...
Text Solution
|
- Which of the following reaction/s give same product?
Text Solution
|
- The correct statements among the following is/are
Text Solution
|
- Which of the following substrates is/are more reactive than ethyl brom...
Text Solution
|
- Choose the correct option(s)
Text Solution
|
- Metallic gold frequently is found in aluminosilicate rocks and it is f...
Text Solution
|
- Metallic gold frequently is found in aluminosilicate rocks and it is f...
Text Solution
|
- X(C(4)H(9)Br) underset(Delta) overset(alc.KOH)to Yoverset(Br(2)//"CC"l...
Text Solution
|
- X(C(4)H(9)Br) underset(Delta) overset(alc.KOH)to Yoverset(Br(2)//"CC"l...
Text Solution
|