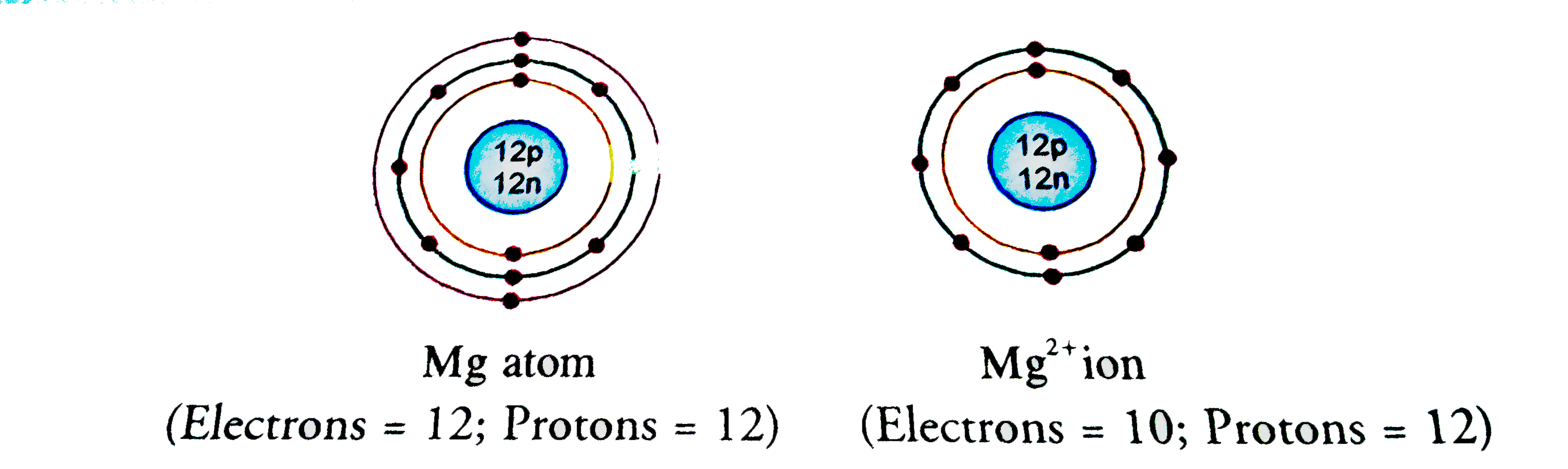Text Solution
Verified by Experts
Topper's Solved these Questions
STRUCTURE OF ATOM
DINESH PUBLICATION|Exercise Long Answer Question|20 VideosSTRUCTURE OF ATOM
DINESH PUBLICATION|Exercise Higher order thinking skilll based questions|5 VideosSTRUCTURE OF ATOM
DINESH PUBLICATION|Exercise Very Short Answer Questions|51 VideosMATTER IN OUR SURROUNDINGS
DINESH PUBLICATION|Exercise (LAQs) Long Answer Questions|5 Videos
Similar Questions
Explore conceptually related problems
DINESH PUBLICATION-STRUCTURE OF ATOM -Short Answer Question
- complete the following table:
Text Solution
|
- An element is represented as overset(16)underset(8)X.Find: (a) The n...
Text Solution
|
- The electronic configuration of potassium (K) is 2,8,8,1 instedad of 2...
Text Solution
|
- Show the electron distribution in magnesium atom and magnesium ion dia...
Text Solution
|
- The composition of two atoms A and B is given: (a) What are the m...
Text Solution
|
- (a) what is the relationship between two elements x and y whose atomic...
Text Solution
|
- write the elctron distrubution of oxygen atom .How many valence elect...
Text Solution
|
- (a) Why does helium have zero valecy? (b) Name the secientist and h...
Text Solution
|
- Which observations in scatteing experiment led Rutherford to make the ...
Text Solution
|
- Write the two postulates of Thomson's model o fan atom .What were the ...
Text Solution
|
- (a) how many neutrons are present in c-14 isotope of carbon? (b) ho...
Text Solution
|
- What information do you get from the figure about the atomic number, m...
Text Solution
|
- In response to a question, a student stated that in an atom, the numbe...
Text Solution
|
- Calculate the number of neutrons present in the nucleus of an element ...
Text Solution
|
- Match the names of the Scientists given in Column A with their contrib...
Text Solution
|
- The atomic number of calcium and argon are 20 and 18 respectively, but...
Text Solution
|
- Complete the table on the basis of information available in the symbol...
Text Solution
|
- Helium atom has 2 electrons in its valence shell but its valency is no...
Text Solution
|
- Fill in the blanks in the following statements: (a) Rutherford's al...
Text Solution
|
- An element X has a mass number 4 and atomic number 2. Write the valenc...
Text Solution
|