Text Solution
Verified by Experts
Topper's Solved these Questions
STRUCTURE OF ATOM
DINESH PUBLICATION|Exercise Higher order thinking skilll based questions|5 VideosSTRUCTURE OF ATOM
DINESH PUBLICATION|Exercise Multiple choice question|26 VideosSTRUCTURE OF ATOM
DINESH PUBLICATION|Exercise Short Answer Question|45 VideosMATTER IN OUR SURROUNDINGS
DINESH PUBLICATION|Exercise (LAQs) Long Answer Questions|5 Videos
Similar Questions
Explore conceptually related problems
DINESH PUBLICATION-STRUCTURE OF ATOM -Long Answer Question
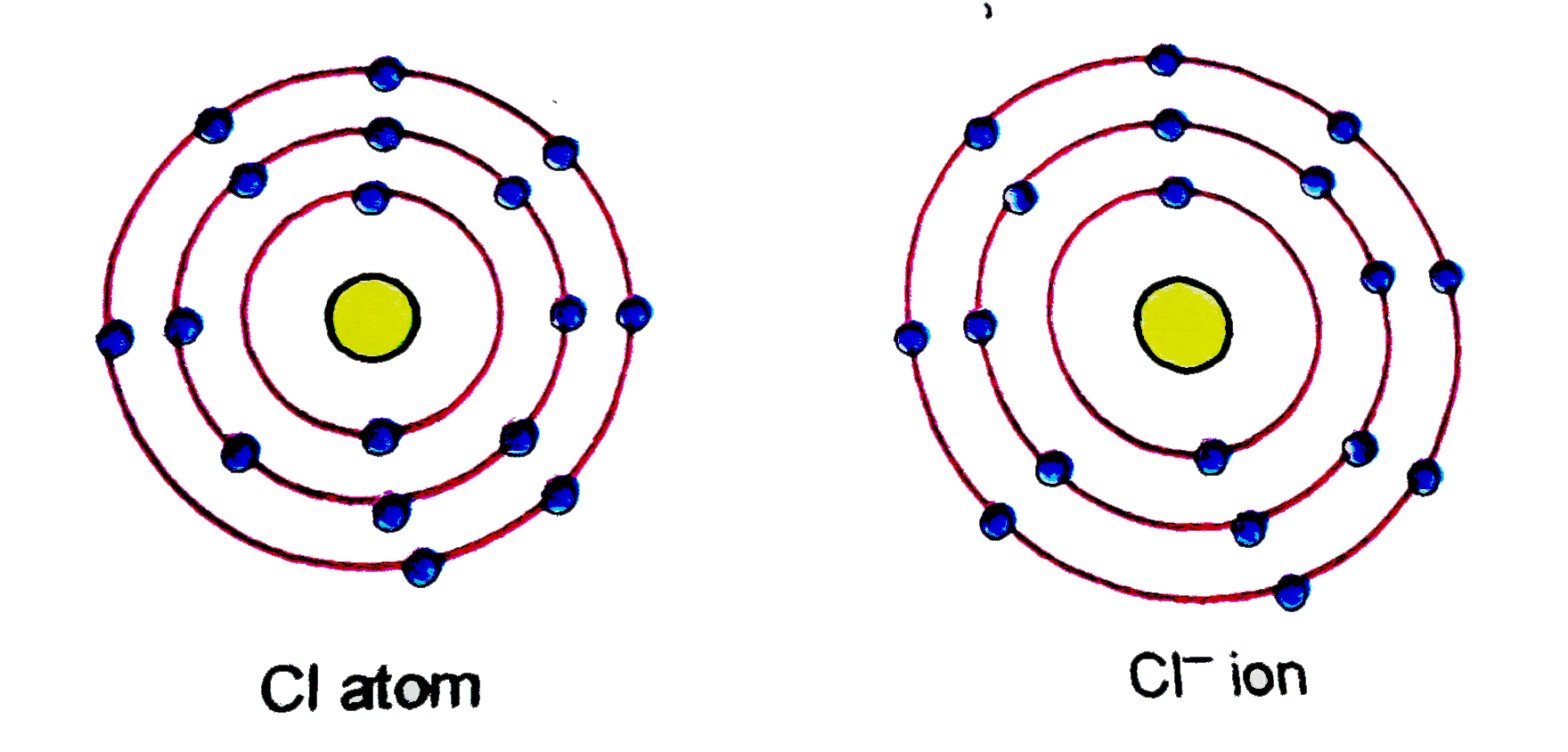
- (a) Give the schematic atomic structures of chlorine atom and chloride...
Text Solution
|
- Answer the following in one line or two: (a) what is the maximum nu...
Text Solution
|
- Which observations in scatteing experiment led Rutherford to make the ...
Text Solution
|
- With the help of suitable acitivies shows that (i) cathode rays trav...
Text Solution
|
- The average atomic mass of a sample of an element X is 16.2 mu. What i...
Text Solution
|
- Give reasons for the following: (a) Isotopes of an element are chemi...
Text Solution
|
- The following data repersents the distribution of electons protons and...
Text Solution
|
- An atom of an elements has two electrons in outermost M shell state it...
Text Solution
|
- (a) why are the chemical properties of the isotopes same? (b) Draw b...
Text Solution
|
- (a) In the gold foil experiment , what observations led Rutherford t...
Text Solution
|
- (a) state the postulates stated by neils bohr in order to overcome the...
Text Solution
|
- Define isotopes why do isotopes have same atomic number but differente...
Text Solution
|
- Why do helium, neon and argon have a zero valency?
Text Solution
|
- The ratio of the radii of hydrogen atom and its nucleus is ~ 10^(5). ...
Text Solution
|
- Enlist the conclusions drawn by Rutherford from his alpha-ray scatteri...
Text Solution
|
- In what way is the Rutherford's atomic model different from that of Th...
Text Solution
|
- What were the drawbacks of Rutherford's model of an atom?
Text Solution
|
- What are the postulates of Bohr's model of an atom?
Text Solution
|
- Show diagramatically the electron distributions in a sodium atom and a...
Text Solution
|
- In the gold foil experiment of Geiger and Marsden, that paved the way ...
Text Solution
|