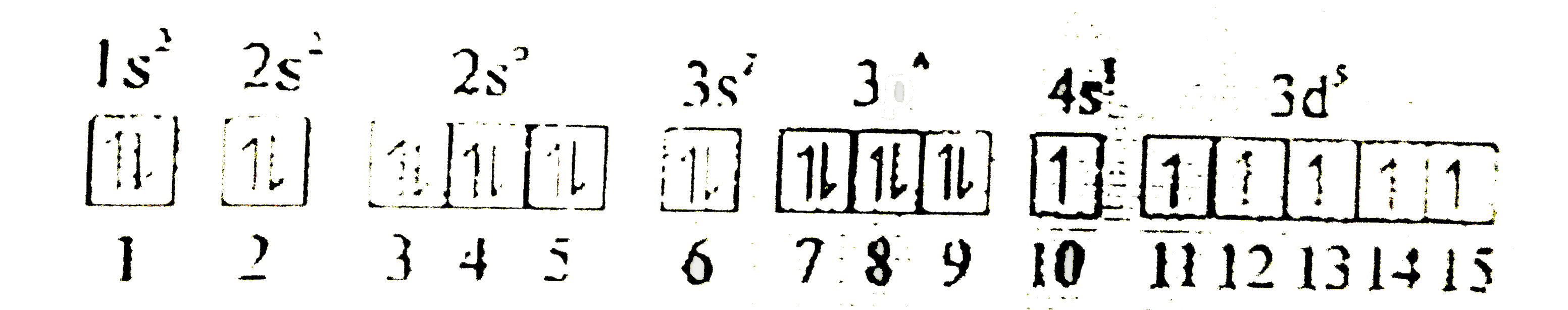Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
BANSAL-TEST PAPERS-CHEMISTRY
- Calculate 'Q' for last electron of Ga. where Q=n+l+ maximum possible...
Text Solution
|
- Calculate total number of orbitals having (n+l) value=8 and magnetic q...
Text Solution
|
- How many orbitals, contains at least one electron in the ground state ...
Text Solution
|
- Calculate the total number of p-orbitals electrons present in Cu(29) a...
Text Solution
|
- Final the total number of species having two unparired electron from t...
Text Solution
|
- What is the mass number of an element A, if A^(4-) contains 10 electro...
Text Solution
|
- Which of the following has maximum number of unpaired electrons:
Text Solution
|
- Which of the following is not a mixture?
Text Solution
|
- Effective nuclear charge on last electron of Fe^(2+) (Using Slater's R...
Text Solution
|
- Which species has highest magnetic moment?
Text Solution
|
- Which of the following is a compound?
Text Solution
|
- Correct set of quantum number for last electron of La (57):
Text Solution
|
- In which transition maximum amount of energy will be released.
Text Solution
|
- Which of the following orbital is non-directed in nature?
Text Solution
|
- d(z)2 orbital is combination of :
Text Solution
|
- Which of the following is impossible set of quantum numbers.
Text Solution
|
- Orbital angular momentum associated with 2p-electron is:
Text Solution
|
- Identify the atom which has ground state configuration [Ar]3d^(10)4s^(...
Text Solution
|
- Name of element with atomic number (z)=110.
Text Solution
|
- Which of the following is exothermic:
Text Solution
|