Text Solution
Verified by Experts
Topper's Solved these Questions
REDOX REACTIONS
CHHAYA PUBLICATION|Exercise SOLVED NCERT EXERCISE|64 VideosREDOX REACTIONS
CHHAYA PUBLICATION|Exercise HIGHER ORDER THINKING SKILLS (HOTS) QUESTIONS|12 VideosREDOX REACTIONS
CHHAYA PUBLICATION|Exercise QUESTION- ANSWER ZONE FOR BOARD EXAMINATION (VERY SHOT ANSWER TYPE)|44 VideosCLASSIFICATION OF ELEMENTS & PERIODICITY IN PROPERTIES
CHHAYA PUBLICATION|Exercise PRACTICE SET|12 Videoss-BLOCK ELEMENTS
CHHAYA PUBLICATION|Exercise PRACTICE SET|16 Videos
Similar Questions
Explore conceptually related problems
CHHAYA PUBLICATION-REDOX REACTIONS -SOLVED WBCHSE SCANNNER
- What are the oxidation number of the two elements marked with asterisk...
Text Solution
|
- Balance the following chemical equation by oxidation number method P...
Text Solution
|
- Balance the following equation by ion-electron method : K(2)Cr(2)O(7...
Text Solution
|
- Balance the following equation by ion-electron method : K(2)Cr(2)O(7...
Text Solution
|
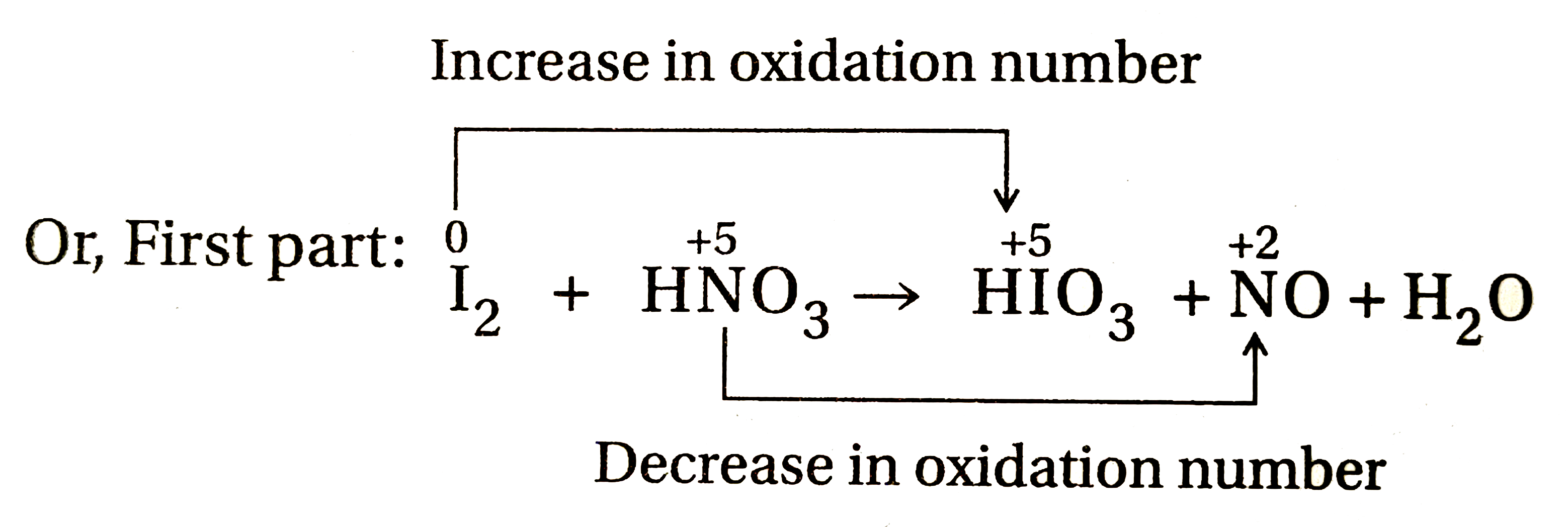
- Balance the following equation by oxidation number method : I(2)+HNO(3...
Text Solution
|
- Balance by ion -electron method : K(3)Cr(2)O(7)+H(2)SO(4)+KItoCr(2)(S...
Text Solution
|
- Balance by oxidation number metod : CU+HNO(3)toCu(NO(3))(2)+NO+H(2)O
Text Solution
|
- Balance the following chemical reaction by oxidation number method : ...
Text Solution
|
- Give an example of compound where the constituent element in exhibits ...
Text Solution
|
- Mention the oxidation number of two chlorine atoms in Ca(OCI)CI molecu...
Text Solution
|
- Balance the following chemical equation by oxidation number method ...
Text Solution
|
- What is the oxidation number of N atom in NaN(3)molecule?
Text Solution
|
- Balance the following chemical equation by ion electron method : MnO...
Text Solution
|
- Calculate the oxidation state of sulphur in H(2)SO(5)
Text Solution
|
- P(4)+3NaOH+3H(2)OtoPH(3)+3NaH(2)PO(2) What is the equivalent weight o...
Text Solution
|
- What is the oxidation sate of Cr in CrO(5)?
Text Solution
|
- Balance the following equation by electron method : MnO(4)^(-1)+SO(2...
Text Solution
|
- What is the oxidation number of Mn in K(2) MnO(4)?
Text Solution
|
- Balance the following chemical equation by ion electron method: Cr2O(7...
Text Solution
|
- What is the oxidation number of S in S(8)?
Text Solution
|