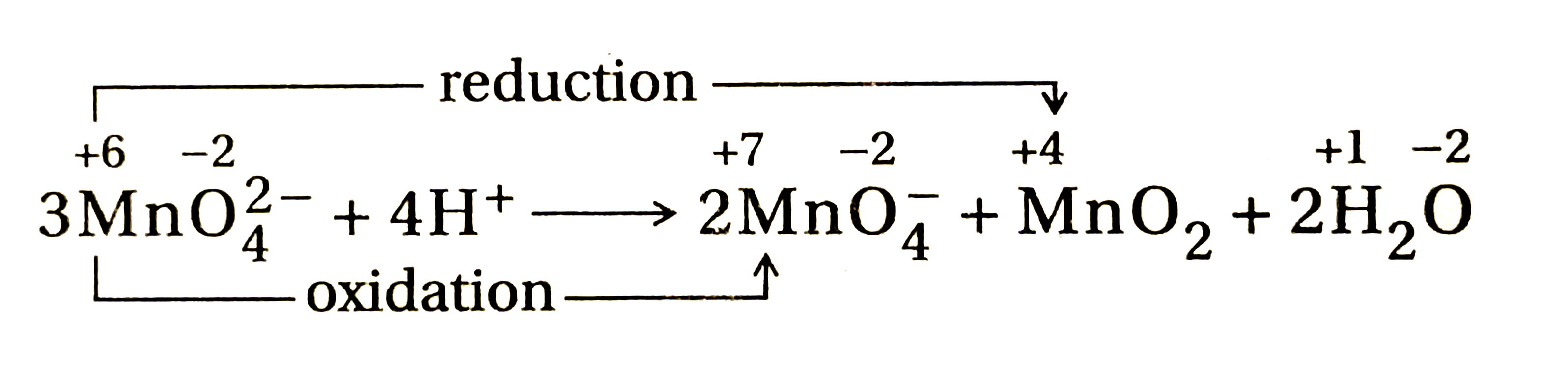Text Solution
Verified by Experts
Topper's Solved these Questions
REDOX REACTIONS
CHHAYA PUBLICATION|Exercise ENTRANCE QUESTION|20 VideosREDOX REACTIONS
CHHAYA PUBLICATION|Exercise MCQ HOTSPOT (SINGLE CORRECT TYPE)|40 VideosREDOX REACTIONS
CHHAYA PUBLICATION|Exercise SOLVED NCERT EXERCISE|64 VideosCLASSIFICATION OF ELEMENTS & PERIODICITY IN PROPERTIES
CHHAYA PUBLICATION|Exercise PRACTICE SET|12 Videoss-BLOCK ELEMENTS
CHHAYA PUBLICATION|Exercise PRACTICE SET|16 Videos
Similar Questions
Explore conceptually related problems
CHHAYA PUBLICATION-REDOX REACTIONS -HIGHER ORDER THINKING SKILLS (HOTS) QUESTIONS
- MnO(4)^(2-)undergoes disproportionation reaction in acidic medium but ...
Text Solution
|
- What amount of K(2)Cr(2)O(7)(in mmol) is required to oxidise 24ml 0.05...
Text Solution
|
- Explain with mechanism why the reaction between O(3)and H(2)O(2) writ...
Text Solution
|
- 12.53cm^(3)0.051MseO(2)reacts completely with 25.5cm^(3)0.1MCrSO(4)to ...
Text Solution
|
- 30 mL 0.05 M KMnO(4) is required for complete oxidation of 0.5 g oxala...
Text Solution
|
- What will be the nature of the salt formed when 2 mol NaOH is added to...
Text Solution
|
- Find the oxidation state of C-1 and C-2 in CH(3)CH(2)OH.
Text Solution
|
- 1 mol N(2)H(4) loses 10 mol of electrons with th electrons with the fo...
Text Solution
|
- Oxidation number of the elements A, B and C +2,+5 and -2 respectively ...
Text Solution
|
- In acidic medium .for the reduction of each NO(3)^(-) ion in the given...
Text Solution
|
- CO(3)O(4) is an oxide of Ca (III) and Cu (II) .If its formula is CO(x)...
Text Solution
|
- How many electrons should A(2)H(3)liberate so that in the new compound...
Text Solution
|