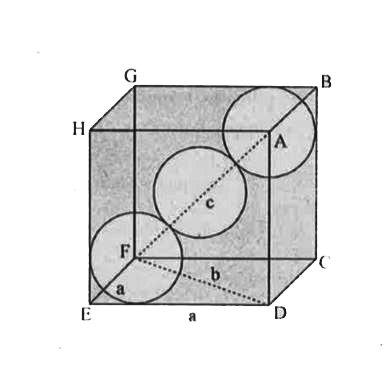
Text Solution
Verified by Experts
Topper's Solved these Questions
EXAM QUESTION PAPER JULY WITH ANSWER (2015)
SUBHASH PUBLICATION|Exercise PART E|21 VideosEXAM QUESTION PAPER JULY WITH ANSWER (2015)
SUBHASH PUBLICATION|Exercise PART C|14 VideosELECTROCHEMISTRY
SUBHASH PUBLICATION|Exercise Problem Section|25 VideosGENERAL PRINCIPLES AND PROCESSES OF ISOLATION OF ELEMENTS
SUBHASH PUBLICATION|Exercise Questions|38 Videos
Similar Questions
Explore conceptually related problems
SUBHASH PUBLICATION-EXAM QUESTION PAPER JULY WITH ANSWER (2015)-PART D
- Calculate the number of particles in Body Centered Cubic (BCC) lattice...
Text Solution
|
- An element having atomic mass 107.9 u has FCC lattice. The edge length...
Text Solution
|
- The boiling point of benzene is 353.23 K when 1.80 g of a non-volatile...
Text Solution
|
- Write two differences between ideal and non-ideal solutions.
Text Solution
|
- Draw the neat lableled diagram of SHE and write its symbolic represent...
Text Solution
|
- Calculate the e.m.f. of the cell in which the following reaction takes...
Text Solution
|
- Derive the integrated rate equation for rate constant of Zero order re...
Text Solution
|
- Show that the rate of first order reaction is doubled when concentrati...
Text Solution
|
- Give any two chracteristics of chemisorption
Text Solution
|
- What is meant by selectivity of a catalyst ?
Text Solution
|
- Define Brownian movement
Text Solution
|
- Define Tyndall effect
Text Solution
|