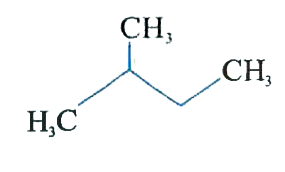
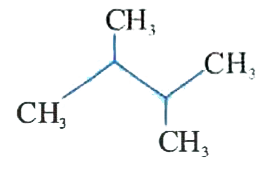
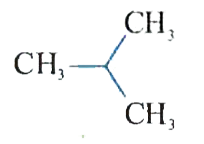
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
HYDROCARBONS
NARAYNA|Exercise EVALUATE YOURSELF - 1|2 VideosHYDROCARBONS
NARAYNA|Exercise EVALUATE YOURSELF - 2|2 VideosHYDROCARBONS
NARAYNA|Exercise EXERCISE - 4|18 VideosGENERAL ORGANIC CHEMISTRY
NARAYNA|Exercise Matrix-Match type questions|7 VideosHYDROGEN & ITS COMPOUCDS
NARAYNA|Exercise Interger type questions|10 Videos
Similar Questions
Explore conceptually related problems
NARAYNA-HYDROCARBONS -EXAMPLE
- Which of the following alkane can be obtained in good yield by the Wur...
Text Solution
|
- A sample of 450 mg of unknown alcohol is added to CH(3)MgBr when 168 o...
Text Solution
|
- The main product produced in the dehydrohalogenation of 2-bromo-3,3-di...
Text Solution
|
- Boverset("i) "BH(3)//THF)underset("ii) "H(2)O(2)//OH)rarr A and Bare ...
Text Solution
|
- When propyne is treated with aqueous H(2)SO(4) in presence of HgSO(4),...
Text Solution
|
- In the reaction Ph-C-=C-CH(3)overset(H(3)O^(+))underset(HgSO(4))rarrA,...
Text Solution
|
- Which of the following is correct order of stability of given radicals...
Text Solution
|
- Among the following compounds (I - III), the correct order of reaction...
Text Solution
|