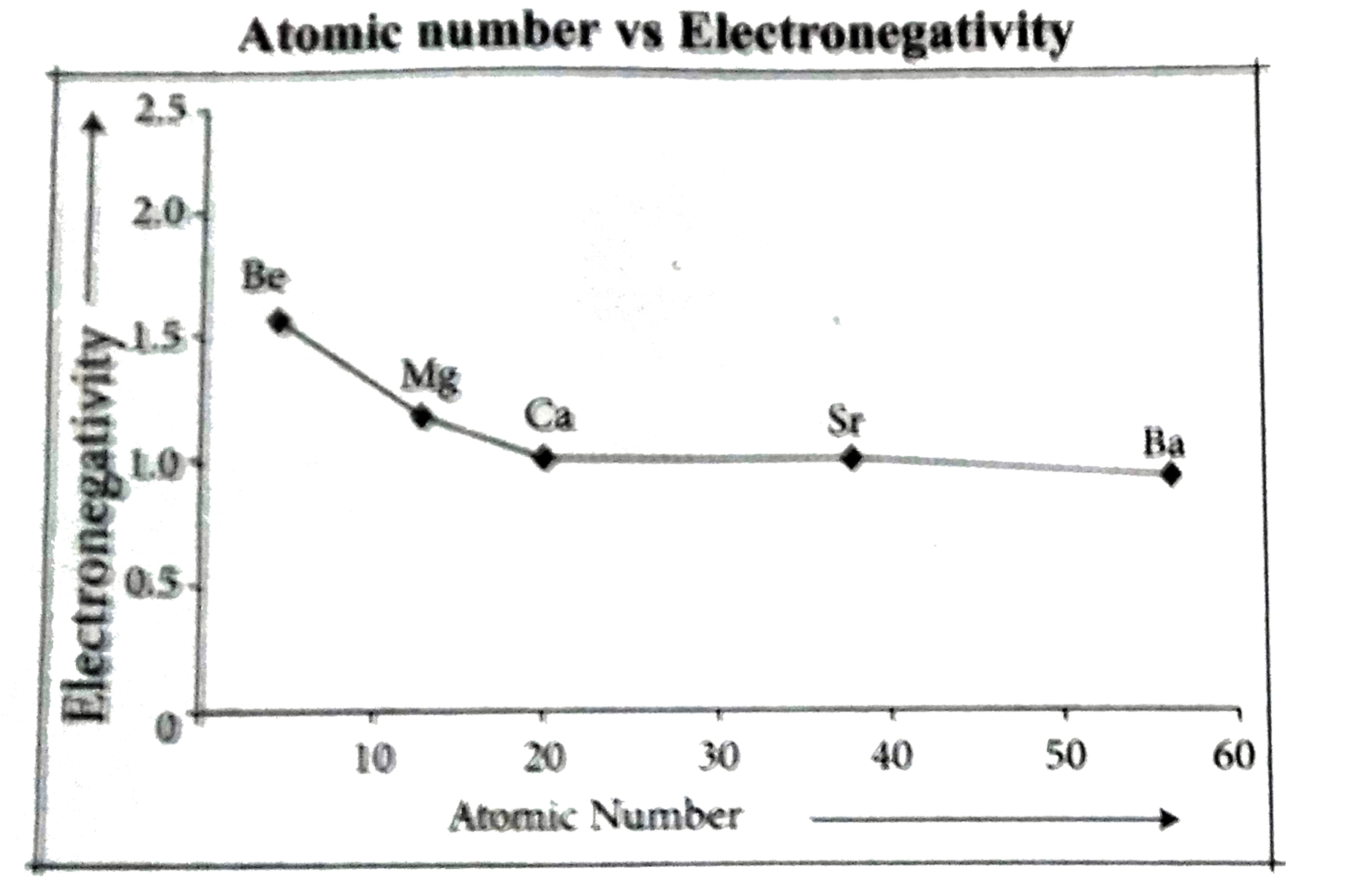
`2^nd` group elements :
`17^th` group elements :
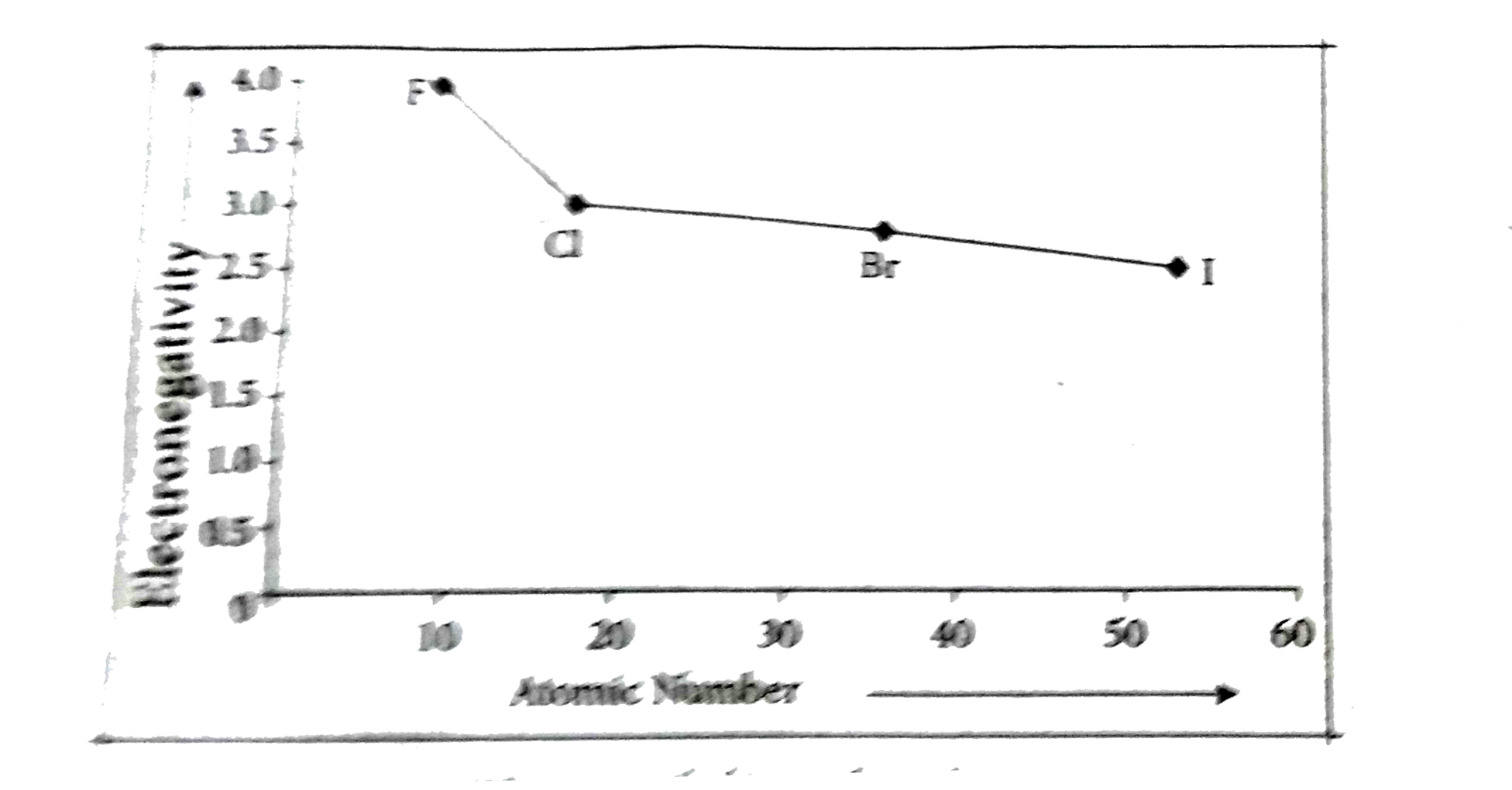
As we go down the group, the electronegativity value decreases. Moving down the group, the electronegativity decreases due to the longer distance between the nucleus and the valence electron shell thereby decreasing the attraction, making the atom have less attraction for electrons or protons.
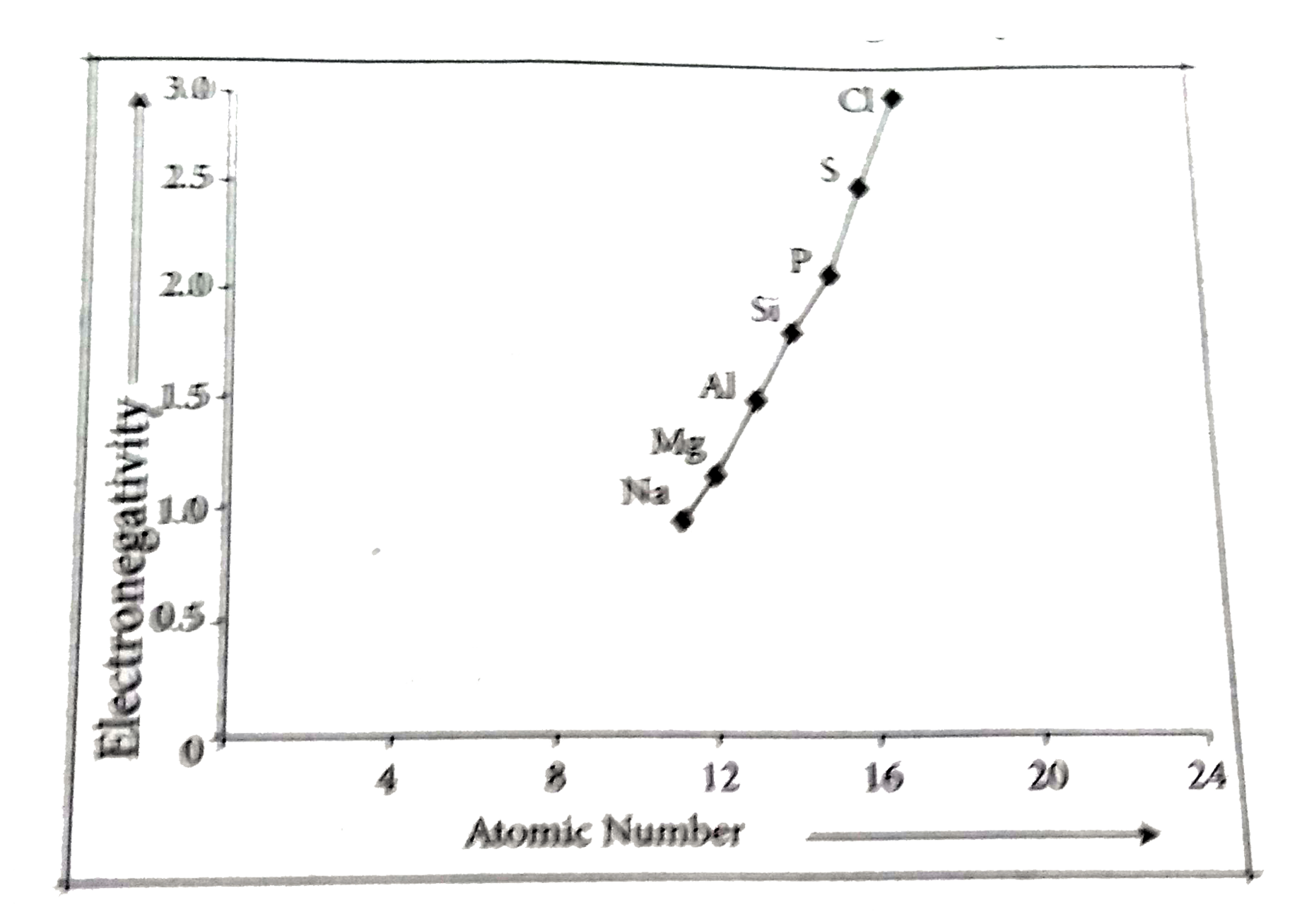
`3^rd` period:
`4^th` period:
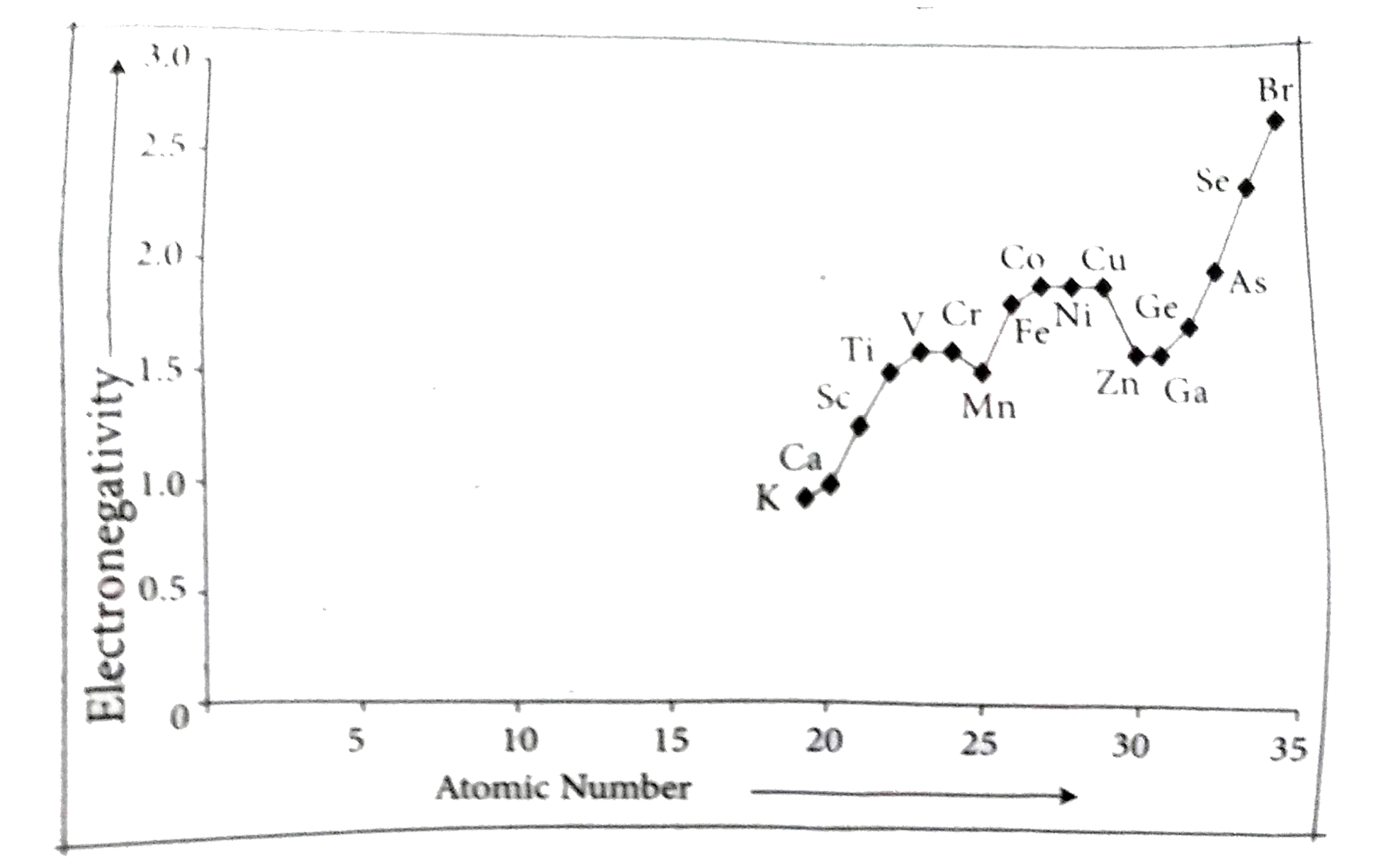
The positively charged protons in the nucleus attract the negatively charged electrons. As the number of protons in the nucleus increases, the electronegativity or attraction will increase. Therefore electronegativity increases from left to right across the period. This occurs due to the greater charge on the nucleus, causing the electron bonding pairs to be very attracted to atoms placed further right on the periodic table.