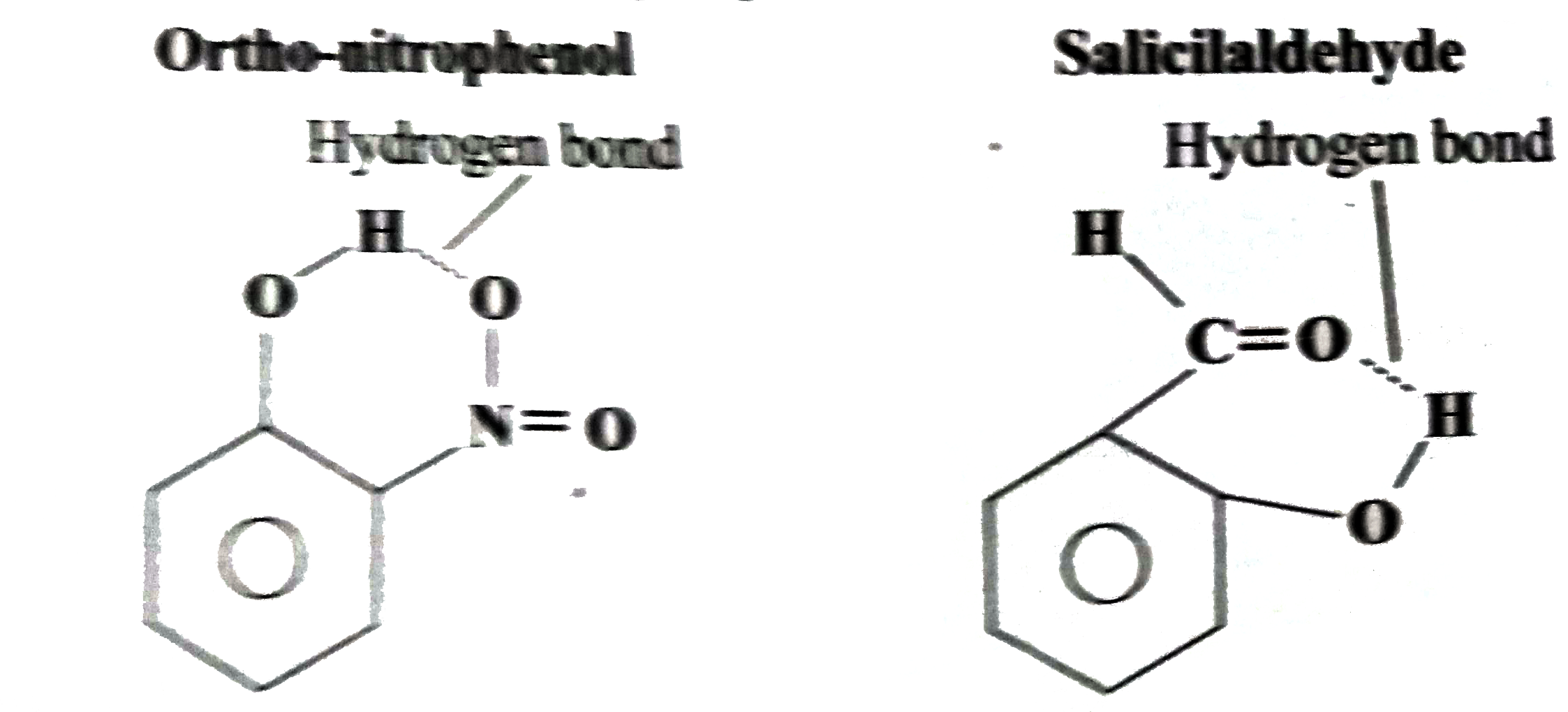Text Solution
Verified by Experts
Topper's Solved these Questions
HYDROGEN
FULL MARKS|Exercise Additional Questions Solved 5-Mark Questions|10 VideosHYDROGEN
FULL MARKS|Exercise Additional Questions Solved 2-Mark Questions|38 VideosHYDROCARBONS
FULL MARKS|Exercise Additional Question Solved (5 mark question )|15 VideosPERIODIC CLASSIFICATION OF ELEMENTS
FULL MARKS|Exercise Activity 3.2|1 Videos
Similar Questions
Explore conceptually related problems
FULL MARKS-HYDROGEN -Additional Questions Solved 3-Mark Questions
- Write a note about ortho water and para water
Text Solution
|
- Water is an amphoteric oxide. Justify this statement.
Text Solution
|
- Distinguish between hard water and soft water
Text Solution
|
- Explain the action of soap with hard water.
Text Solution
|
- Describe about ion exchange method of softening water (or) Explain Zeo...
Text Solution
|
- Explain about the exchange reactions of deuterium oxide.
Text Solution
|
- Complete the following reactions. AI(4)C(3)+D(2)Oto? CaC(2)+D(2)Oto...
Text Solution
|
- What are the uses of hydrogen peroxide?
Text Solution
|
- Prove that H(2)O(2) act as reducing agent in alkaline medium.
Text Solution
|
- Write a note about saline (or) ionic hydride.
Text Solution
|
- What are metallic hydrides? Explain about it.
Text Solution
|
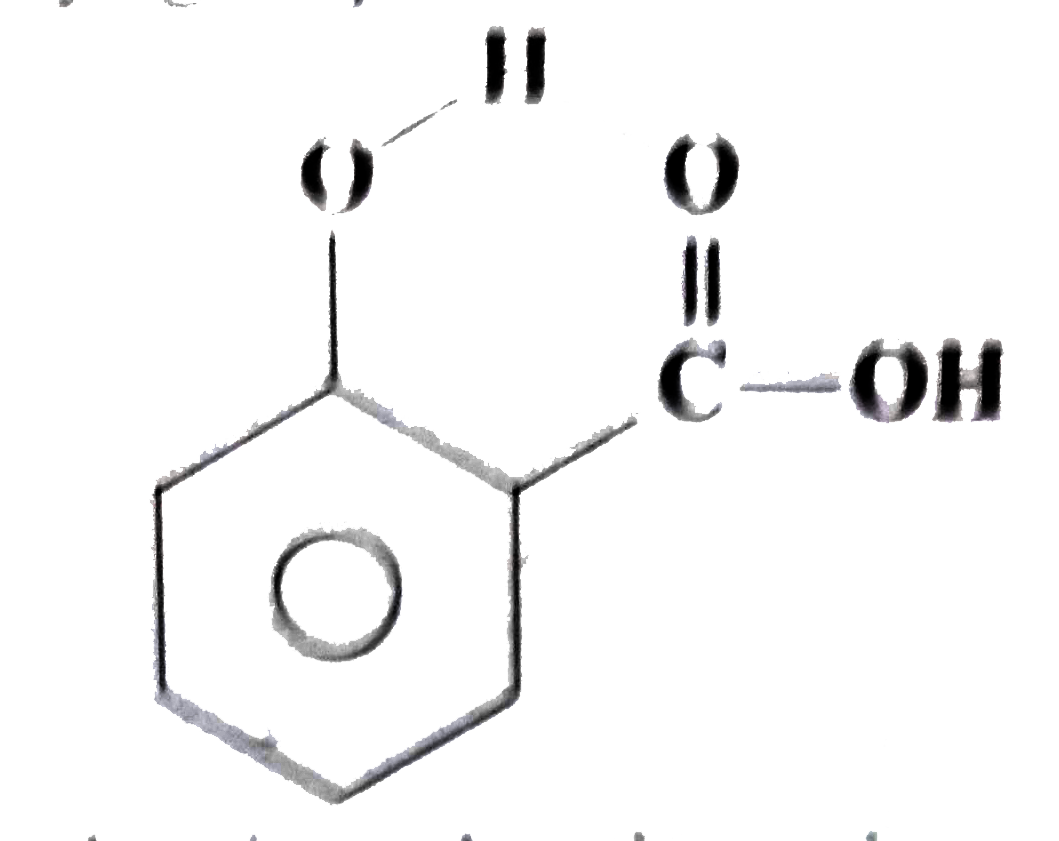
- What are intermolecular hydrogen bonds? Explain with example.
Text Solution
|
- What are intermolecular hydrogen bonds? Explain with example.
Text Solution
|
- Explain about the importance of hydrogen bonding in proteins.
Text Solution
|
- What are Clathrate hydrate? Explain it with suitable example.
Text Solution
|
- What are crystalline hydrates? Explain it with example.
Text Solution
|
- What do you understand by (i) Electron-deficient (ii) Electron-precise...
Text Solution
|
- What causes the temporary and permanent hardness of water?
Text Solution
|
- Write the chemical reactions to show the amphoteric nature of water.
Text Solution
|
- What is the difference between the terms 'hydrolysis' and 'hydration'?...
Text Solution
|