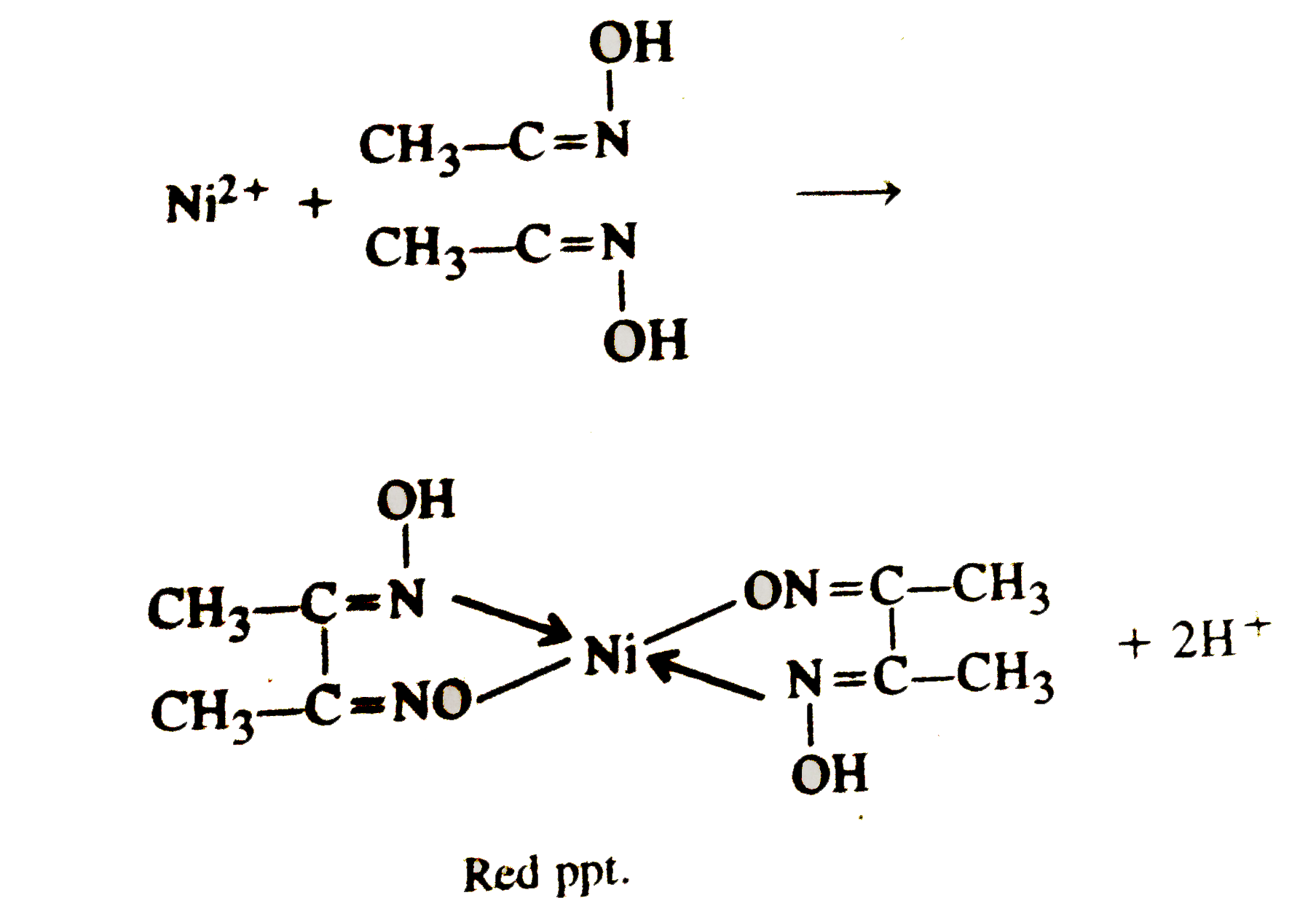
A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
PRINCIPLES RELATED TO PRACTICAL CHEMISTRY
DINESH PUBLICATION|Exercise Brain teasers-34|1 VideosPRINCIPLES RELATED TO PRACTICAL CHEMISTRY
DINESH PUBLICATION|Exercise Brain teasers-35|1 VideosPRINCIPLES RELATED TO PRACTICAL CHEMISTRY
DINESH PUBLICATION|Exercise Brain teasers-32|1 VideosPOLYMERS
DINESH PUBLICATION|Exercise Matrix|5 VideosPURIFICATION & CHARACTERISATION OF ORGANIC COMPOUND
DINESH PUBLICATION|Exercise Ultimate Preparatory Package|15 Videos
Similar Questions
Explore conceptually related problems