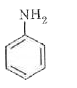
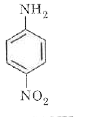
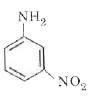
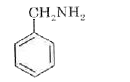
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
ORGANIC COMPOUNDS CONTAINING NITROGEN
MODERN PUBLICATION|Exercise REVISION EXERCISES PASSAGE BASED QUESTIONS|10 VideosORGANIC COMPOUNDS CONTAINING NITROGEN
MODERN PUBLICATION|Exercise REVISION EXERCISES ASSERTION REASON QUESTIONS|10 VideosORGANIC COMPOUNDS CONTAINING NITROGEN
MODERN PUBLICATION|Exercise QUICK MEMORY TEST C. CHOOSE THE CORRECT ALTERNATIVE|10 VideosHALOALKANES AND HALOARENES
MODERN PUBLICATION|Exercise UNIT PRACTICE TEST|11 VideosPOLYMERS
MODERN PUBLICATION|Exercise Competition file (OBJECTIVE TYPE QUESTIONS) (C. MULTIPLE CHOICE QUESTIONS)(Integer Type Questions)|6 Videos
Similar Questions
Explore conceptually related problems
MODERN PUBLICATION-ORGANIC COMPOUNDS CONTAINING NITROGEN -REVISION EXERCISES (OBJECTIVE TYPE QUESTIONS) (MULTIPLE CHOICE QUESTIONS)
- Which of the following statement is incorrect?
Text Solution
|
- When primary amine reacts with choloroform in ethanolic KOH then the p...
Text Solution
|
- Which of the following does not react with Hinsberg reagent?
Text Solution
|
- C(6)H(5)N(2)Cl + CuCN to C(6)H(5)CN + N(2) + CuCl is
Text Solution
|
- Which of the following is most basic?
Text Solution
|
- Among the following which one is strongest base?
Text Solution
|
- Which of the following compound is the most basic?
Text Solution
|
- The IUPAC name of is
Text Solution
|
- Gabriel phthalimide synthesis is used in the preparation of
Text Solution
|
- Which of the following compound will be formed when aniline reacts wit...
Text Solution
|
- C(2)H(5)NH(2) + HNO(2) to A, A is :
Text Solution
|
- underset(Delta)overset(H(3)O^(+))(to) P, P is :
Text Solution
|
- CH(3)C -= N overset(H(2) // Ni)(to) P, P will be?
Text Solution
|
- Which of the following compound gives dye test ?
Text Solution
|
- The IUPAC name of the compound H(3)C-CH(2)-underset(CH(2))underset(|...
Text Solution
|
- Complete the following reaction
Text Solution
|
- Match the following columns
Text Solution
|
- Match the following columns
Text Solution
|
- Match the following columns
Text Solution
|
- Match the following columns
Text Solution
|