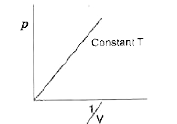
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
STATES OF MATTER : GASES AND LIQUIDS
MODERN PUBLICATION|Exercise COMPETITION FILE OBJECTIVE TYPE QUESTIONS (D. MULTIPLE CHOICE QUESTIONS)|7 VideosSTATES OF MATTER : GASES AND LIQUIDS
MODERN PUBLICATION|Exercise COMPETITION FILE OBJECTIVE TYPE QUESTIONS (D. MULTIPLE CHOICE QUESTIONS)(Matrix Match Type Question)|2 VideosSTATES OF MATTER : GASES AND LIQUIDS
MODERN PUBLICATION|Exercise COMPETITION FILE OBJECTIVE TYPE QUESTIONS (B. MULTIPLE CHOICE QUESTIONS)(JEE (ADVANCED) FOR IIT ENTRANCE)|8 VideosSOME BASIC CONCEPTS OF CHEMISTRY
MODERN PUBLICATION|Exercise COMPETITION FILE (INTEGER TYPE AND NUMERICAL VALUE TYPE QUESTIONS)|10 VideosSTRUCTURE OF ATOM
MODERN PUBLICATION|Exercise Unit Practice Test|13 Videos
Similar Questions
Explore conceptually related problems
MODERN PUBLICATION-STATES OF MATTER : GASES AND LIQUIDS-COMPETITION FILE OBJECTIVE TYPE QUESTIONS (C. MULTIPLE CHOICE QUESTIONS)
- Which of the following statements is // are correct ?
Text Solution
|
- Which of the following plots are correct for an ideal gas ?
Text Solution
|
- Which of the following statements is not correct?
Text Solution
|
- VAN DER WAALS EQUATION
Text Solution
|
- A gas described by van der Waals equation .
Text Solution
|
- Which of the following changes decrease the vapour pressure of water k...
Text Solution
|
- Under which of the following conditions applied together, a gas deviat...
Text Solution
|
- According to kinetic theory of gases:
Text Solution
|
- Two gases X (mol. wt. M(X)) and Y (mol. wt. M(Y) , M(Y) gt M(X)) are a...
Text Solution
|
- Which of the following statement(s) is (are) are correct regarding the...
Text Solution
|