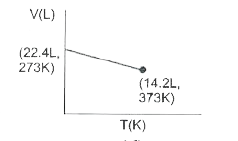A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
STATES OF MATTER : GASES AND LIQUIDS
MODERN PUBLICATION|Exercise COMPETITION FILE OBJECTIVE TYPE QUESTIONS (C. MULTIPLE CHOICE QUESTIONS)|10 VideosSTATES OF MATTER : GASES AND LIQUIDS
MODERN PUBLICATION|Exercise COMPETITION FILE OBJECTIVE TYPE QUESTIONS (D. MULTIPLE CHOICE QUESTIONS)|7 VideosSTATES OF MATTER : GASES AND LIQUIDS
MODERN PUBLICATION|Exercise COMPETITION FILE OBJECTIVE TYPE QUESTIONS (B. MULTIPLE CHOICE QUESTIONS)(JEE (MAIN) & OTHER STATE BOARDS FOR ENGINEERING ENTRANCE)|44 VideosSOME BASIC CONCEPTS OF CHEMISTRY
MODERN PUBLICATION|Exercise COMPETITION FILE (INTEGER TYPE AND NUMERICAL VALUE TYPE QUESTIONS)|10 VideosSTRUCTURE OF ATOM
MODERN PUBLICATION|Exercise Unit Practice Test|13 Videos
Similar Questions
Explore conceptually related problems
MODERN PUBLICATION-STATES OF MATTER : GASES AND LIQUIDS-COMPETITION FILE OBJECTIVE TYPE QUESTIONS (B. MULTIPLE CHOICE QUESTIONS)(JEE (ADVANCED) FOR IIT ENTRANCE)
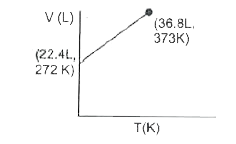
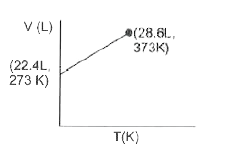
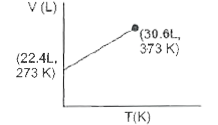
- Which of the following volume-temperature (V-I) plots represents the b...
Text Solution
|
- Positive deviation from ideal behaviour takes place because of
Text Solution
|
- The root mean square velocity of one mole of a monoatomic gas having m...
Text Solution
|
- The ratio of the rate of diffusion of helium and methane under indenti...
Text Solution
|
Text Solution
|
- The term that corrects for the attractive forces present in a real gas...
Text Solution
|
- For one mole of a van der Waals' gas when b=0 and T=300K, the pV vs 1/...
Text Solution
|
- The equalitative sketches I, II and III given below show the variation...
Text Solution
|