Text Solution
Verified by Experts
Topper's Solved these Questions
ELECTRO CHEMISTRY
FULL MARKS|Exercise EVALUATE YOURSELF|7 VideosELECTRO CHEMISTRY
FULL MARKS|Exercise ADDITIONAL QUESTIONS - CHOOSE THE BEST ANSWER AND WRITE IT|110 VideosELECTRO CHEMISTRY
FULL MARKS|Exercise TEXTBOOK EVALUATION - CHOOSE THE CORRECT ANSWER|25 VideosCOORDINATION CHEMISTRY
FULL MARKS|Exercise Additional Question(5 Marks Question)|26 VideosHYDROXY COMPOUNDS AND ETHERS
FULL MARKS|Exercise ADDITIONAL QUESTIONS (Answer the following)|115 Videos
Similar Questions
Explore conceptually related problems
FULL MARKS-ELECTRO CHEMISTRY-TEXTBOOK EVALUATION - SHORT ANSWER
- Why does conductivity of a solution decrease on dilution of the soluti...
Text Solution
|
- State Kohirausch Law. How is it useful to determine the molar conducti...
Text Solution
|
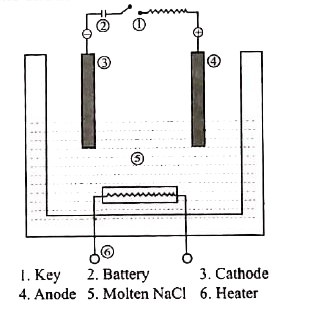
- Describe the electrolysis of molten NaCl using inert electrodes.
Text Solution
|
- State Faraday's Laws of electrolysis.
Text Solution
|
- Describe the construction of Daniel cell. Write the cell reaction.
Text Solution
|
- Why is anode is galvanic cell considered to be negative and cathode po...
Text Solution
|
- The conductivity of a 0.01 M solution of a 1:1 weak electrolyte at 298...
Text Solution
|
- Whichof 0.1M HCl and 0.1 M KCl do you expect to have greater molar con...
Text Solution
|
- Arrange the following solution solutions in the decreasing order of sp...
Text Solution
|
- Why is AC current used instead of DC in measuring the electrolytic con...
Text Solution
|
- 0.01 M NaCl solution is placed in two different cells having cell cons...
Text Solution
|
- A current of 1.608 A is passed through 250 mL of 0.5 M solution of cop...
Text Solution
|
- Can Fe^(3+) oxidise Bromide to bromine under standard conditions ? G...
Text Solution
|
- Is it possible to store copper sulphate in an iron vessel for a long t...
Text Solution
|
- Two metals M(1) and M(2) have reduction potential values of -xV and +y...
Text Solution
|
- Calculate the standard emf of the cell : Cd|Cd^(2+)|Cu^(2+)|Cu and det...
Text Solution
|
- In fuel cell H2 and O2 react to produce electricity . In the process, ...
Text Solution
|
- 0.1 M copper sulphate solution in which copper electrode is dipped at ...
Text Solution
|
- For the cell Mg(s) |Mg^(2+)(aq)||Ag^(+)(aq)|Ag (s), calculate the equi...
Text Solution
|
- 8.2 xx 10^(12) litres of water is available in a lake. A power reactor...
Text Solution
|