Text Solution
Verified by Experts
|
Topper's Solved these Questions
AROMATIC COMPOUNDS AND ALKYL AND ARYL HALIDES
CENGAGE CHEMISTRY|Exercise Exercises Concept Application Type|2 VideosView PlaylistAROMATIC COMPOUNDS AND ALKYL AND ARYL HALIDES
CENGAGE CHEMISTRY|Exercise Exercises Concept Application|4 VideosView PlaylistAROMATIC COMPOUNDS AND ALKYL AND ARYL HALIDES
CENGAGE CHEMISTRY|Exercise Exercises Subjective Type|4 VideosView PlaylistAPPENDIX INORGANIC VOLUME 2
CENGAGE CHEMISTRY|Exercise Short Answer Type|179 VideosView PlaylistBIOMOLECULES
CENGAGE CHEMISTRY|Exercise Exercises Archives (Analytical And Descriptive)|8 VideosView Playlist
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY-AROMATIC COMPOUNDS AND ALKYL AND ARYL HALIDES -Exercises Subjective
- Give the chemical test to distinguish between (a) Hexane (I), MeCH=C...
Text Solution
|
Playing Now - Give the product of E2 reaction of the following compounds with atc. K...
Text Solution
|
Play - Draw the diastermers of 2- chloro -1,3- dimethyl cyclohexane and indi...
Text Solution
|
Play - Draw the diastermers of 2- chloro -1,3- dimethyl cyclohexane and indi...
Text Solution
|
Play - (W) and (X) are optically active isomers of C(5) H(9) Cl (W) on treatm...
Text Solution
|
Play - An organic compound (A), C(8)H(4) O(3) in dry benzene in the presene ...
Text Solution
|
Play - Three isomeric hydrocations C(9) H(12) [(A),(B),(C)] oxide to C(9) H(...
Text Solution
|
Play - An organtiic compound (A) contains C = 92.3% and H = 7.7% Its vapour d...
Text Solution
|
Play - An aromatic hydrocarbobn (A) containing C = 91.3% and H = 8.7% on tre...
Text Solution
|
Play - Compound C(8)H(9)Cl (A) on treatment with KICN followedd by hydrolysi...
Text Solution
|
Play - ON disuphonatioon followed by fusion with NaOH an dacidificant, an aro...
Text Solution
|
Play - The compoiund (A0 is xylene. On sulphonation, it gives only one produ...
Text Solution
|
Play - An organci compound C(6) H(4) O(2) NCl (A) reacts with a,la,oo tp gove...
Text Solution
|
Play - A hydrocarbon C(9) H(10) (A) rapidly decolourises cold aquous permanga...
06:25
|
Play - On nuclear chlorination, C(8), H(10) (A) gives a product (B) which may...
05:49
|
Play - When boromobenze is monochlorinated. Two ismoeic compounds (A) and (B)...
04:19
|
Play - 0.450 gm of an aromatic organic compound (A) on ignitin gives 0.905 g...
Text Solution
|
Play

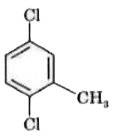 (vii) `CH_(3)-underset(CH_(3))underset(|)overset(CH_(3))overset(|)C-Cl`
(vii) `CH_(3)-underset(CH_(3))underset(|)overset(CH_(3))overset(|)C-Cl`