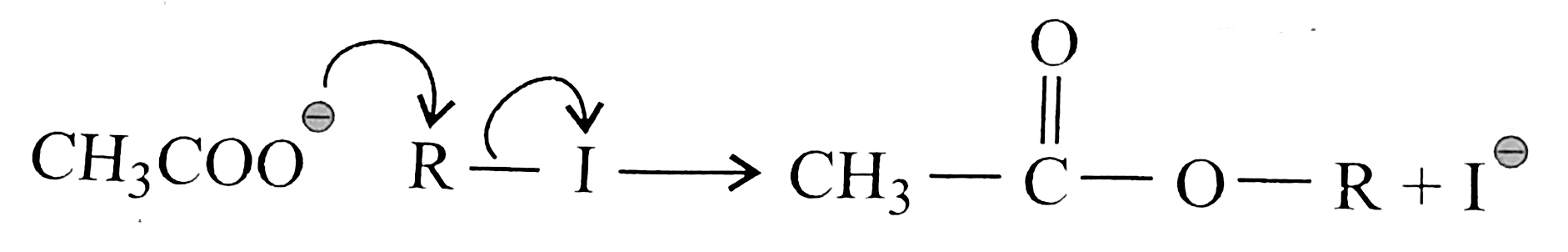Text Solution
Verified by Experts
|
Topper's Solved these Questions
AROMATIC COMPOUNDS AND ALKYL AND ARYL HALIDES
CENGAGE CHEMISTRY|Exercise Exercises Concept Application|4 VideosView PlaylistAROMATIC COMPOUNDS AND ALKYL AND ARYL HALIDES
CENGAGE CHEMISTRY|Exercise Exercises Linked Compreshension Type|11 VideosView PlaylistAROMATIC COMPOUNDS AND ALKYL AND ARYL HALIDES
CENGAGE CHEMISTRY|Exercise Exercises Subjective|17 VideosView PlaylistAPPENDIX INORGANIC VOLUME 2
CENGAGE CHEMISTRY|Exercise Short Answer Type|179 VideosView PlaylistBIOMOLECULES
CENGAGE CHEMISTRY|Exercise Exercises Archives (Analytical And Descriptive)|8 VideosView Playlist
Similar Questions
Explore conceptually related problems
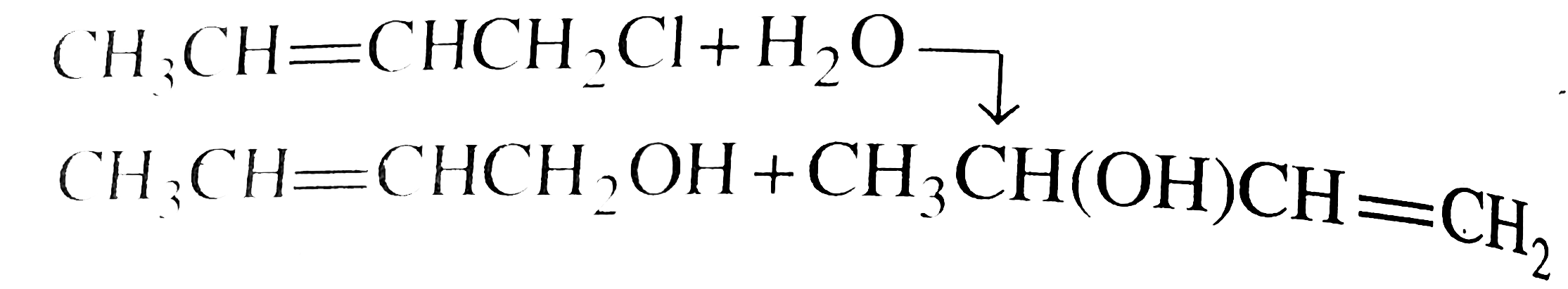

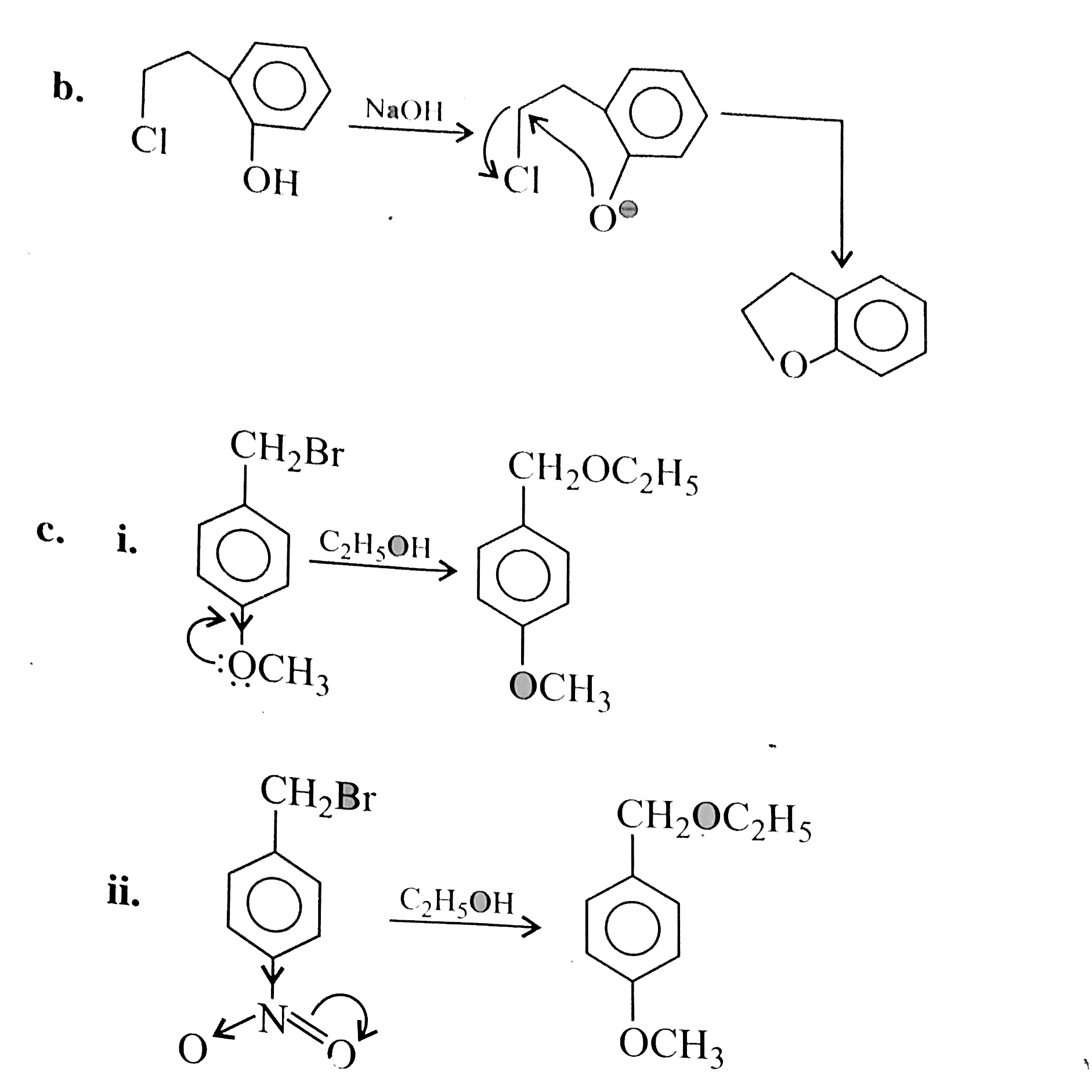
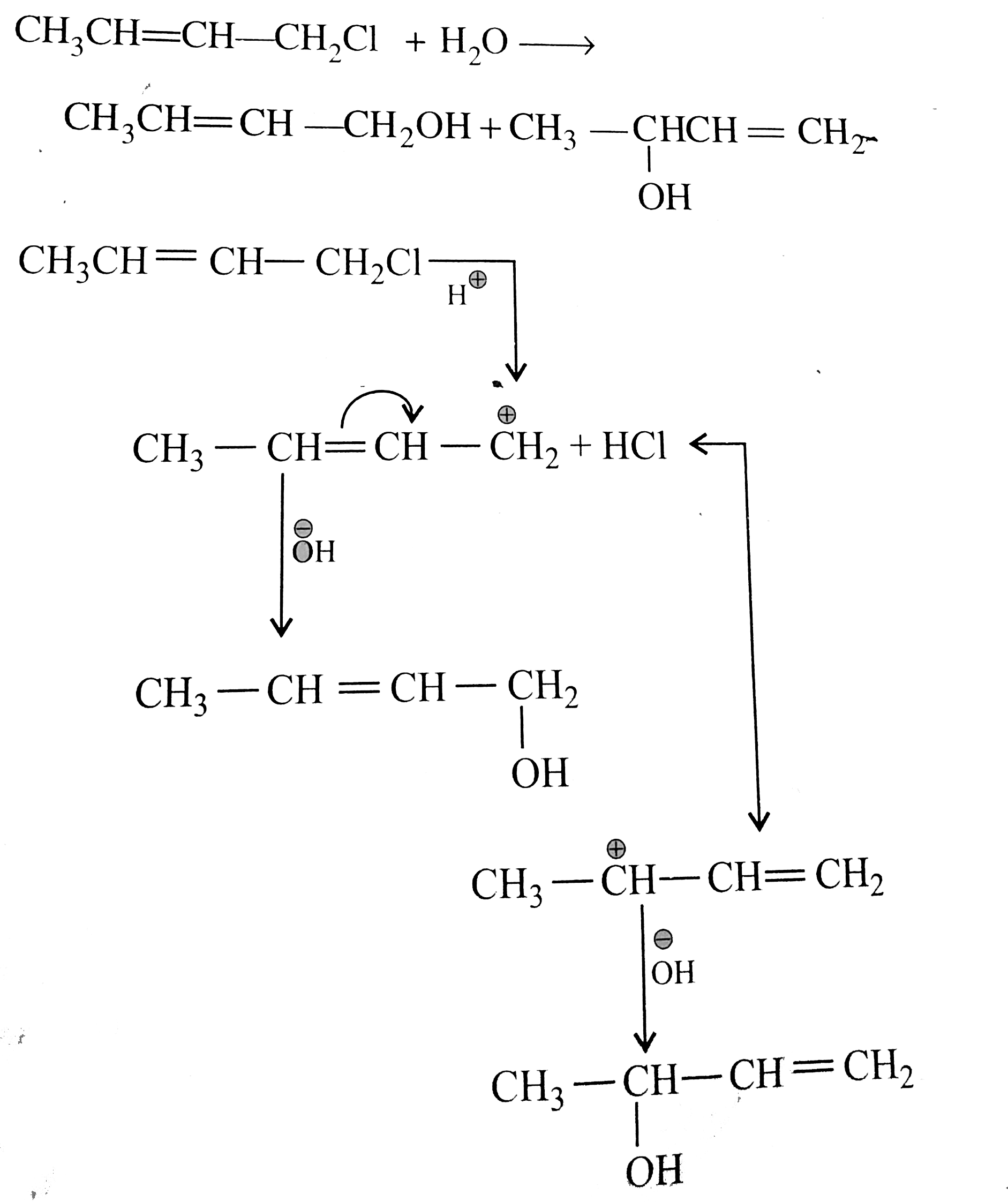
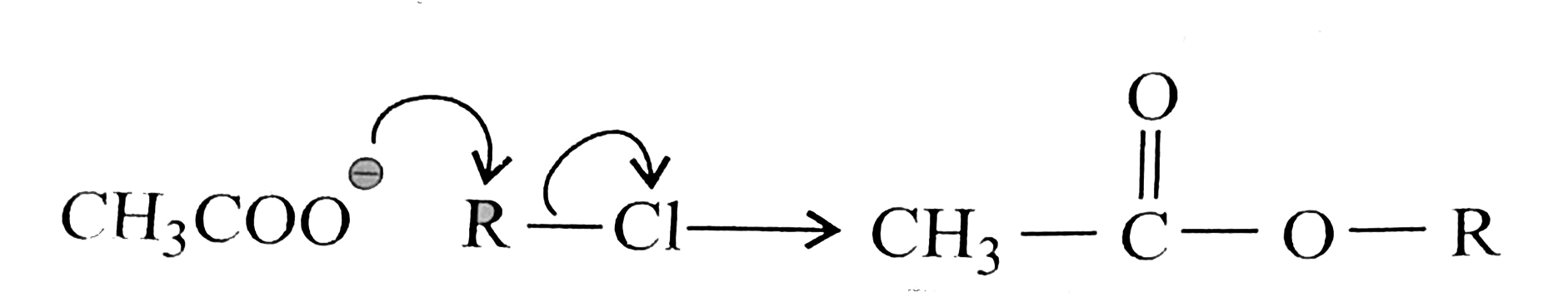 But in alkyl chloride, `(C-Cl)` is a strong bond and is difficult to break. But in the presence of `Nal, (R-Cl)` is converted to `(R-I)` (Finkelstein reaction) and therefore, in `(R-1)` the `(C-1)` bond is weak, which can e easily cleaved. So `SN^(2)` reaction is cataysed in the presence of `Nal`.
But in alkyl chloride, `(C-Cl)` is a strong bond and is difficult to break. But in the presence of `Nal, (R-Cl)` is converted to `(R-I)` (Finkelstein reaction) and therefore, in `(R-1)` the `(C-1)` bond is weak, which can e easily cleaved. So `SN^(2)` reaction is cataysed in the presence of `Nal`.