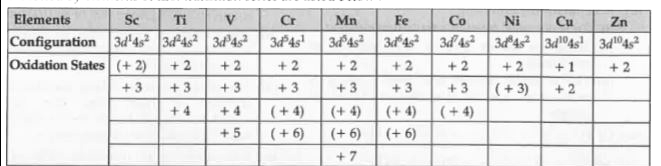
Text Solution
Verified by Experts
Topper's Solved these Questions
D - BLOCK ELEMENTS F - BLOCK ELEMENTS
OSWAAL PUBLICATION|Exercise TOPIC - 2 (F - BLOCK ELEMENT , LANTHANOIDS AND ACTINOIDS)(VERY SHORT ANSWER TYPES QUESTIONS)|17 VideosD - BLOCK ELEMENTS F - BLOCK ELEMENTS
OSWAAL PUBLICATION|Exercise TOPIC - 2 (F - BLOCK ELEMENT , LANTHANOIDS AND ACTINOIDS)(SHORT ANSWER TYPES QUESTIONS)|15 VideosD - BLOCK ELEMENTS F - BLOCK ELEMENTS
OSWAAL PUBLICATION|Exercise TOPIC-1 (D - BLOCK ELEMENTS, THEIR PROPERTIES AND COMPONDS) LONG ANSWER TYPE QUESTIONS - I|20 VideosCO-ORDINATION COMPOUNDS
OSWAAL PUBLICATION|Exercise TOPIC-2 (WERNER.S THEORY BONDING IN CO-ORDINATION COMPOUND , VBT, CFT AND IMPORTANCE OF CO-ORDINATION COMPOUNDS) (Very Answer Type Questions)|19 VideosELECTRO-CHEMISTRY
OSWAAL PUBLICATION|Exercise Topic - 3 ELECTROLYSIS , LAWS OF ELECTROLYSIS, BATTERIES, FUEL CELLS AND CORROSION (LONG ANSWER TYPE QUESTIONS)|4 Videos
Similar Questions
Explore conceptually related problems
OSWAAL PUBLICATION-D - BLOCK ELEMENTS F - BLOCK ELEMENTS -TOPIC-1 (D - BLOCK ELEMENTS, THEIR PROPERTIES AND COMPONDS)LONG ANSWER TYPE QUESTIONS - II
- Describe an experiment to show the effect of concentration on the rate...
Text Solution
|
- Describe experiment to determine the mass of KMnO4 present in 1dm^3 of...
Text Solution
|
- For the estimation of potassium permangnate using standard ferrous amm...
Text Solution
|
- For the estimation of potassium permangnate using standard ferrous amm...
Text Solution
|
- Describe the experimental procedure and give the calculations involved...
Text Solution
|
- (i) Name the element of 3d-transition series which shows maximum numbe...
Text Solution
|
- (a) Complete the following equations: (i) Cr2O(7)^(2-) + 2OH^(-) to ...
Text Solution
|
- (i) With reference to structural variability and chemical reactivity, ...
Text Solution
|
- Give reasons: (i) Zirconium (Z = 40) and Hafnium (Z = 72) have almos...
Text Solution
|
- Describe the preparation of potassium permanganate from pyrolusite ore...
Text Solution
|
- (a) Give reasons for the following observations: (i) Cu^(+) ion is n...
Text Solution
|
- (a) Describe the following characteristics of the first series (Sc to ...
Text Solution
|