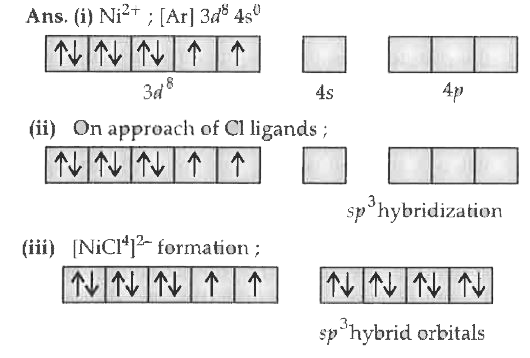
Text Solution
Verified by Experts
Topper's Solved these Questions
CO-ORDINATION COMPOUNDS
OSWAAL PUBLICATION|Exercise TOPIC-2 (WERNER.S THEORY BONDING IN CO-ORDINATION COMPOUND , VBT, CFT AND IMPORTANCE OF CO-ORDINATION COMPOUNDS) (Short Answer Type Questions)|9 VideosCHEMISTY IN EVERYDAY LIFE
OSWAAL PUBLICATION|Exercise LONG ANSWER TYPE QUESTIONS-I|1 VideosD - BLOCK ELEMENTS F - BLOCK ELEMENTS
OSWAAL PUBLICATION|Exercise TOPIC - 2 (F - BLOCK ELEMENT , LANTHANOIDS AND ACTINOIDS)(LONG ANSWER TYPES QUESTIONS)|6 Videos
Similar Questions
Explore conceptually related problems
OSWAAL PUBLICATION-CO-ORDINATION COMPOUNDS-TOPIC-2 (WERNER.S THEORY BONDING IN CO-ORDINATION COMPOUND , VBT, CFT AND IMPORTANCE OF CO-ORDINATION COMPOUNDS) (Very Answer Type Questions)
- Using valence bond theory (VBT), account for the geometry, type of hyb...
Text Solution
|
- With the help of valence bond theory account for the geometry and magn...
Text Solution
|
- Write any three postulates of Werner's theory of complexes.
Text Solution
|
- Using VBT, explainthe geometry and magnetic property of [Ni(CN)(4)]^(-...
Text Solution
|
- Using valence bond theory explain geometry, hybridisation and magnetic...
Text Solution
|
- Mention any two applications of co-ordination compounds.
Text Solution
|
- What is crystal field splitting?
Text Solution
|
- Salient features of Molecules Orbital Theory (MOT)
Text Solution
|
- With the help of VBT explain the hybridisation in tetracarbonyl nickel...
Text Solution
|
- Discuss briefly giving an example in each case the role of co-ordinati...
Text Solution
|
- Aqueous copper sulphate solution (blue in colour) gives (i) a green pr...
Text Solution
|
- Explain the hybridisation, geometry and magnetic property of [Ni(Cl)(4...
Text Solution
|
- Which of the following overlaping of atomic orbital will not be allowe...
Text Solution
|
- On the basis of Valence bond theory account for the hybridization, sha...
Text Solution
|
- With the help of VBT explain the geometry of K4 [Fe(CN)6 and predict i...
Text Solution
|
- What is meant by stability of a co-ordination compound in solution ? S...
Text Solution
|
- What is crystal field splitting energy ? How does the magnitude of Del...
Text Solution
|
- Just like human beings, plants also need various nutrients for their h...
Text Solution
|
- Who is the father of co-ordination chemistry?
Text Solution
|