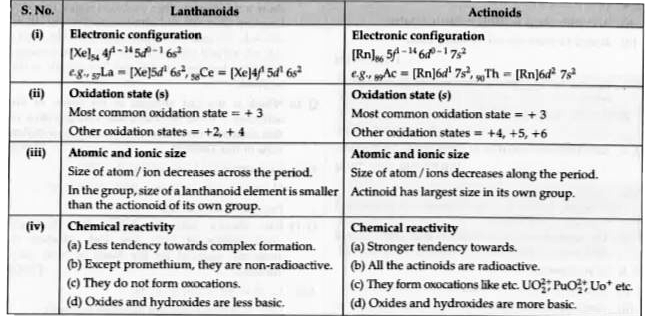
Text Solution
Verified by Experts
Topper's Solved these Questions
D - BLOCK ELEMENTS F - BLOCK ELEMENTS
OSWAAL PUBLICATION|Exercise TOPIC - 2 (F - BLOCK ELEMENT , LANTHANOIDS AND ACTINOIDS)(SHORT ANSWER TYPES QUESTIONS)|15 VideosCO-ORDINATION COMPOUNDS
OSWAAL PUBLICATION|Exercise TOPIC-2 (WERNER.S THEORY BONDING IN CO-ORDINATION COMPOUND , VBT, CFT AND IMPORTANCE OF CO-ORDINATION COMPOUNDS) (Very Answer Type Questions)|19 VideosELECTRO-CHEMISTRY
OSWAAL PUBLICATION|Exercise Topic - 3 ELECTROLYSIS , LAWS OF ELECTROLYSIS, BATTERIES, FUEL CELLS AND CORROSION (LONG ANSWER TYPE QUESTIONS)|4 Videos
Similar Questions
Explore conceptually related problems
OSWAAL PUBLICATION-D - BLOCK ELEMENTS F - BLOCK ELEMENTS -TOPIC - 2 (F - BLOCK ELEMENT , LANTHANOIDS AND ACTINOIDS)(LONG ANSWER TYPES QUESTIONS)
- Compare the chemistry with that of the lanthanoids with special refere...
Text Solution
|
- Give examples and suggest reasons for the following features of the tr...
Text Solution
|
- How would you account for the following: (i) Among lanthanoids, Ln (...
Text Solution
|
- How would you account for the following: (i) Among lanthanoids, Ln (...
Text Solution
|
- Compare the chemistry with that of the lanthanoids with special refere...
Text Solution
|
- Give some uses of (application) of d- and f-block elements.
Text Solution
|