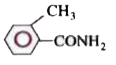
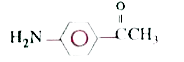
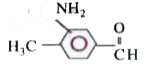
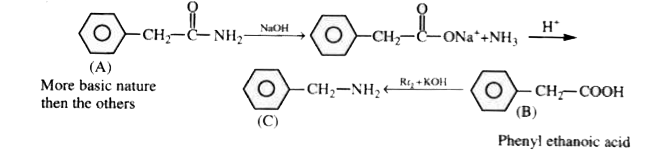
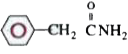A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CARBOXYLIC ACIDS
AAKASH SERIES|Exercise PRACTICE SHEET-1 (Matching Type Questions)|2 VideosCARBOXYLIC ACIDS
AAKASH SERIES|Exercise PRACTICE SHEET-1 (Interger Type Questions)|7 VideosCARBOXYLIC ACIDS
AAKASH SERIES|Exercise PRACTICE SHEET-1 (More than one correct answer questions)|5 VideosCARBOHYDRATES
AAKASH SERIES|Exercise Practice Sheet-5|30 VideosCARBOXYLIC ACIDS AND DERIVATIVES
AAKASH SERIES|Exercise CONVERSIONS|19 Videos
Similar Questions
Explore conceptually related problems
AAKASH SERIES-CARBOXYLIC ACIDS -PRACTICE SHEET-1 (Linked comprehension Type Questions)
- An organic compound (A) of molecular weight 135 on boiling with NaOH e...
Text Solution
|
- An organic compound (A) of molecular weight 135 on boiling with NaOH e...
Text Solution
|
- An organic compound (A) of molecular weight 135 on boiling with NaOH e...
Text Solution
|
- Compound A(C8 H7 OCl) on reaction with one equivalent of CH3 MgBr gave...
Text Solution
|
- Compound A(C8 H7 OCl) on reaction with one equivalent of CH3 MgBr gave...
Text Solution
|
- Compound A(C8 H7 OCl) on reaction with one equivalent of CH3 MgBr gave...
Text Solution
|