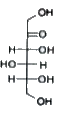
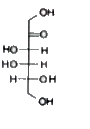
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
AAKASH SERIES-CARBOHYDRATES-Practice Sheet-5
- D-tagatose is a 2-ketohexose which forms same osazone as D-galactose. ...
Text Solution
|
- The correct chair form structures of beta- galactose will be
Text Solution
|
- Killiani-Fischer synthesis of D (+) Arabinose gives
Text Solution
|
- An optically active compound A gave an [alpha](0)^(25) = 30^(@) while ...
Text Solution
|
- Although D-galactose rotates plane polarized light its oxidation produ...
Text Solution
|
- underset(underset(CH(2)OH)(|))overset(overset(overset(overset(CHO)(|))...
Text Solution
|
- Find the average molecular mass of starch given that an aqueous soluti...
Text Solution
|
- Which of the following orders of sweetness is correct
Text Solution
|
- In the multi step conversion of an Aldose in to next higher Aldose by ...
Text Solution
|
- The Fischer projection formula shown above is the enolic form of
Text Solution
|
- The correct chair form of given Haworth Structure will be
Text Solution
|
- The product of the reaction D-Glyceraldehyde underset(HCl)overset(Me(2...
Text Solution
|
- Which relationship (s)is/are true
Text Solution
|
- Which of the following monosaccharides yields an optically active aldi...
Text Solution
|
- Observe the following reaction and mark the correct statements(s)given...
Text Solution
|
- Which of the following statements are correct
Text Solution
|
- Monosaccharides have -CHO (or C=O) and -OH groups, so they undergo usu...
Text Solution
|
- Monosaccharides have -CHO (or C=O) and -OH groups, so they undergo usu...
Text Solution
|
- Monosaccharides have -CHO (or C=O) and -OH groups, so they undergo usu...
Text Solution
|
- What is true about compound (I)
Text Solution
|