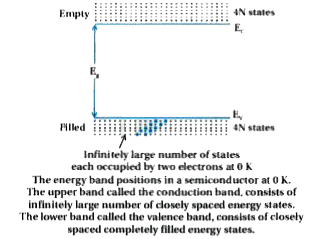
Text Solution
Verified by Experts
Topper's Solved these Questions
SEMICONDUCTOR ELECTRONICS : MATERIALS, DEVICES AND SIMPLE CIRCUITS
KUMAR PRAKASHAN|Exercise Section-B : Numericals (Numerical From Textual illustrations)|16 VideosSEMICONDUCTOR ELECTRONICS : MATERIALS, DEVICES AND SIMPLE CIRCUITS
KUMAR PRAKASHAN|Exercise Section-B : Numericals (Numerical From Textual Exercise)|15 VideosSEMICONDUCTOR ELECTRONICS : MATERIALS, DEVICES AND SIMPLE CIRCUITS
KUMAR PRAKASHAN|Exercise Section-D : Multiple Choice Questions (MCQs) (MCQs asked in Competitive Exams)|129 VideosSAMPLE QUESTION PAPER
KUMAR PRAKASHAN|Exercise PART-B SECTION-C|5 VideosWAVE OPTICS
KUMAR PRAKASHAN|Exercise SECTION-D (MULTIPLCE CHOICE QUESTIONS (MCQS)) (MCQS FROM DARPAN BASED ON TEXTBOOK)|239 Videos
Similar Questions
Explore conceptually related problems
KUMAR PRAKASHAN-SEMICONDUCTOR ELECTRONICS : MATERIALS, DEVICES AND SIMPLE CIRCUITS -Section-A : Questions - Answers
- Why the motion of electron in solid and in an isolated atom is differe...
Text Solution
|
- Write the definition of valence band and conduction band.
Text Solution
|
- Explain by drawing the energy levels of Si and Ge containing N atoms a...
Text Solution
|
- Explain conductor (metal), insulator and semiconductor by drawing diag...
Text Solution
|
- Explain the crystalline structure by writing the electronic configurat...
Text Solution
|
- Explain the concept of the hole in the semiconductor.
Text Solution
|
- Explain with diagram, how current flows due to electron and hole in pu...
Text Solution
|
- Explain the change in the band with the temperature of the intrinsic ...
Text Solution
|
- Why is it required to add impurity in pure semiconductor? Mention it's...
Text Solution
|
- What is n-type semiconductor? Name the majority and minority carriers ...
Text Solution
|
- What is p-type semiconductor? Name the majority and minority carriers ...
Text Solution
|
- Explain n and p-type semiconductor based on band theory.
Text Solution
|
- Explain recombination coefficient of intrinsic semiconductor and obtai...
Text Solution
|
- Write four points in distinguishing between p-type semiconductor and n...
Text Solution
|
- Write the difference between intrinsic and extrinsic semiconductor.
Text Solution
|
- Which is the primary constitutional unit for diode and transistor?
Text Solution
|
- How p-n junction diode is formed ? And explain depletion layer and bar...
Text Solution
|
- What is depletion barrier?
Text Solution
|
- Write short on semiconductor diode.
Text Solution
|
- State the methods of connection of p-n junction.
Text Solution
|