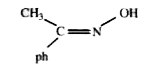
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ISOMERISM
AAKASH SERIES|Exercise PRACTICE SHEET EXERCISE-II (Stereo Isomerism) LEVEL-II (ADVANCED) More than One correct answer Type Questions|5 VideosISOMERISM
AAKASH SERIES|Exercise PRACTICE SHEET EXERCISE-II (Stereo Isomerism) LEVEL-II (ADVANCED) Linked Comprehension Type Questions (Passage - I)|3 VideosISOMERISM
AAKASH SERIES|Exercise PRACTICE SHEET EXERCISE-II (Stereo Isomerism) LEVEL-I (MAIN) Straight Objective Type Questions|18 VideosIONIC EQUILIBRIUM
AAKASH SERIES|Exercise ADDITIONAL PRACTICE EXERCISE (LEVEL -II PRACTICE SHEET (ADVANCED) (Integer Type Questions))|8 VideosPERIODIC CLASSIFICATION
AAKASH SERIES|Exercise OBJECTIVE EXERCISE - 3 (RECENT AIPMT/NEET QUESTIONS)|14 Videos
Similar Questions
Explore conceptually related problems
AAKASH SERIES-ISOMERISM -PRACTICE SHEET EXERCISE-II (Stereo Isomerism) LEVEL-II (ADVANCED) Straight Objective Type Questions
- The number of cis-trans isomer possible for the following compound
Text Solution
|
- Which of the following have zero dipole moment?
Text Solution
|
- Which one of the following will not show geometrical isomerism ?
Text Solution
|
- Geometrical isomerism is possible in:
Text Solution
|
- Select the systematic name for
Text Solution
|
- Tautomer of which of the following can show geometrical isomerism
Text Solution
|
- Which of the following statements is not true regarding the cis and tr...
Text Solution
|
- Which of the following pairs of compounds are geometrical isomers
Text Solution
|
- is
Text Solution
|
- Regarding geometrical isomers which is not correct
Text Solution
|
- No.of geometrical isomers possible for the compound CH(3)-CH=CH-CH=CH-...
Text Solution
|
- Geometrical isomerism is not shown by
Text Solution
|
- Find out the total number of isomers of possible for C(2)H(2)F(2)
Text Solution
|
- Which among the following is E-Isomer ?
Text Solution
|
- Which among the following is Z-Isomer ?
Text Solution
|
- Determine which of the following compounds is a trans isomer ?
Text Solution
|
- Which of the following statement is correct regarding this structure ?
Text Solution
|
- Which of the following statement is correct regarding this structure ?
Text Solution
|
- Which of the following molecule is E- isomer ?
Text Solution
|
- In the given structure
Text Solution
|