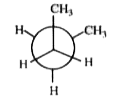
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ISOMERISM
AAKASH SERIES|Exercise PRACTICE SHEET EXERCISE-II (Stereo Isomerism) LEVEL-II (ADVANCED) Straight Objective Type Questions|23 VideosISOMERISM
AAKASH SERIES|Exercise PRACTICE SHEET EXERCISE-II (Stereo Isomerism) LEVEL-II (ADVANCED) More than One correct answer Type Questions|5 VideosISOMERISM
AAKASH SERIES|Exercise PRACTICE SHEET EXERCISE-I (Structural Isomerism) LEVEL-II (ADVANCED) Integer Type Questions|3 VideosIONIC EQUILIBRIUM
AAKASH SERIES|Exercise ADDITIONAL PRACTICE EXERCISE (LEVEL -II PRACTICE SHEET (ADVANCED) (Integer Type Questions))|8 VideosPERIODIC CLASSIFICATION
AAKASH SERIES|Exercise OBJECTIVE EXERCISE - 3 (RECENT AIPMT/NEET QUESTIONS)|14 Videos
Similar Questions
Explore conceptually related problems
AAKASH SERIES-ISOMERISM -PRACTICE SHEET EXERCISE-II (Stereo Isomerism) LEVEL-I (MAIN) Straight Objective Type Questions
- The dihedral angle between the two methyl groups in gauche conformatio...
Text Solution
|
- The dihedral angle between the two methyl groups in anti conformation ...
Text Solution
|
- The number of conformational isomers possible of n-butane about C(2)-C...
Text Solution
|
- Match List-I with List-II and select the correct option.
Text Solution
|
- Consider the following conformations of 3-Aminopropanal, amongst the g...
Text Solution
|
- Most stable carbocation among the following is
Text Solution
|
- Sino-atrial node is present in
Text Solution
|
- Which of the following is associated with Torsional strain ?
Text Solution
|
- Among the structure shown below, which has lowest potential energy?
Text Solution
|
- Increasing order of stability among the three main conformation (i.e. ...
Text Solution
|
- In which of the following has minimum torsional strain and minimum Van...
Text Solution
|
- Which of the following pairs of compounds are geometrical isomers
Text Solution
|
- The cause of cis-trans isomerism is
Text Solution
|
- Which of the following compounds exhibits geometrical isomerism? und...
Text Solution
|
- Which of the following is classified as Z and trans-isomer?
Text Solution
|
- Geometrical isomerism results because molecules has :
Text Solution
|
- Which of the following can show geometrical isomerism
Text Solution
|
- Which of the following orders of stability is correct among these two ...
Text Solution
|