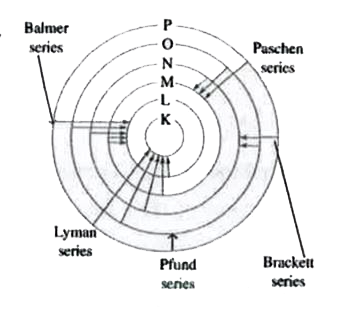
The only electron in the hydrogen atom resides under ordinary conditions on the first orbit. When energy is supplied, the electron moves to higher energy orbit depending on the amount of energy absorbed. When this electron returns to any of the lower orbits, it emits energy. Lyman series is formed when the electron returns to the lowest orbit while Balmer series is formed when the electron returns to second orbit. Similarly, Paschen, Brackett and Pfund series are formed when electron returns to the third, fourth orbits from higher energy orbits respectively (as shown in figure)
Maximum number of lines produced when an electron jumps from nth level to ground level is equal to `(n(n-1))/(2)`. For example, in the case of n = 4, number of lines produced is 6. `(4 rarr 3, 4 rarr 2, 4 rarr 1, 3 rarr 2, 3 rarr 1, 2 rarr 1)`. When an electron returns from `n_(2)` to `n_(1)` state, the number of lines in the spectrum will be equal to `((n_(2) - n_(1))(n_(2)-n_(1) +1))/(2)`
If the electron comes back from energy level having energy `E_(2)` to energy level having energy `E_(2)` then the difference may be expressed in terms of energy of photon as `E_(2) - E_(1) = Delta E, lambda = (h c)/(Delta E)`. Since h and c are constant, `Delta E` corresponds to definite energy, thus each transition from one energy level to another will prouce a higher of definite wavelength. THis is actually observed as a line in the spectrum of hydrogen atom. Wave number of the line is given by the formula `bar(v) = RZ^(2)((1)/(n_(1)^(2)) - (1)/(n_(2)^(2)))`
Where R is a Rydberg constant `(R = 1.1 xx 10^(7))`
(i) First line of a series : it is called .line of logest wavelength. or .line of shortest energy..
(ii) Series limit of last of a series : It is the line of shortest wavelength or line of highest energy.
The wave number of electromagnetic radiation emitted during the transition of electron in between two levels of `Li^(2+)` ion whose principal quantum numbers sum if 4 and difference is 2 is
The only electron in the hydrogen atom resides under ordinary conditions on the first orbit. When energy is supplied, the electron moves to higher energy orbit depending on the amount of energy absorbed. When this electron returns to any of the lower orbits, it emits energy. Lyman series is formed when the electron returns to the lowest orbit while Balmer series is formed when the electron returns to second orbit. Similarly, Paschen, Brackett and Pfund series are formed when electron returns to the third, fourth orbits from higher energy orbits respectively (as shown in figure)
Maximum number of lines produced when an electron jumps from nth level to ground level is equal to `(n(n-1))/(2)`. For example, in the case of n = 4, number of lines produced is 6. `(4 rarr 3, 4 rarr 2, 4 rarr 1, 3 rarr 2, 3 rarr 1, 2 rarr 1)`. When an electron returns from `n_(2)` to `n_(1)` state, the number of lines in the spectrum will be equal to `((n_(2) - n_(1))(n_(2)-n_(1) +1))/(2)`
If the electron comes back from energy level having energy `E_(2)` to energy level having energy `E_(2)` then the difference may be expressed in terms of energy of photon as `E_(2) - E_(1) = Delta E, lambda = (h c)/(Delta E)`. Since h and c are constant, `Delta E` corresponds to definite energy, thus each transition from one energy level to another will prouce a higher of definite wavelength. THis is actually observed as a line in the spectrum of hydrogen atom. Wave number of the line is given by the formula `bar(v) = RZ^(2)((1)/(n_(1)^(2)) - (1)/(n_(2)^(2)))`
Where R is a Rydberg constant `(R = 1.1 xx 10^(7))`
(i) First line of a series : it is called .line of logest wavelength. or .line of shortest energy..
(ii) Series limit of last of a series : It is the line of shortest wavelength or line of highest energy.

The wave number of electromagnetic radiation emitted during the transition of electron in between two levels of `Li^(2+)` ion whose principal quantum numbers sum if 4 and difference is 2 is
Maximum number of lines produced when an electron jumps from nth level to ground level is equal to `(n(n-1))/(2)`. For example, in the case of n = 4, number of lines produced is 6. `(4 rarr 3, 4 rarr 2, 4 rarr 1, 3 rarr 2, 3 rarr 1, 2 rarr 1)`. When an electron returns from `n_(2)` to `n_(1)` state, the number of lines in the spectrum will be equal to `((n_(2) - n_(1))(n_(2)-n_(1) +1))/(2)`
If the electron comes back from energy level having energy `E_(2)` to energy level having energy `E_(2)` then the difference may be expressed in terms of energy of photon as `E_(2) - E_(1) = Delta E, lambda = (h c)/(Delta E)`. Since h and c are constant, `Delta E` corresponds to definite energy, thus each transition from one energy level to another will prouce a higher of definite wavelength. THis is actually observed as a line in the spectrum of hydrogen atom. Wave number of the line is given by the formula `bar(v) = RZ^(2)((1)/(n_(1)^(2)) - (1)/(n_(2)^(2)))`
Where R is a Rydberg constant `(R = 1.1 xx 10^(7))`
(i) First line of a series : it is called .line of logest wavelength. or .line of shortest energy..
(ii) Series limit of last of a series : It is the line of shortest wavelength or line of highest energy.

The wave number of electromagnetic radiation emitted during the transition of electron in between two levels of `Li^(2+)` ion whose principal quantum numbers sum if 4 and difference is 2 is
A
R
B
`(R)/(3^(2))`
C
`(3^(2)R)/(4^(2))`
D
`3^(2)R`
Text Solution
Verified by Experts
The correct Answer is:
A
Topper's Solved these Questions
ATOMIC STRUCTURE
AAKASH SERIES|Exercise Level - II (Type-I) (Practice sheet - 1 (Section -C : Matching/Straight objective type questions))|2 VideosATOMIC STRUCTURE
AAKASH SERIES|Exercise Level - II (Type-I) (Lecture Sheet - 2 (Section-A : More than one correct answer type questions))|5 VideosATOMIC STRUCTURE
AAKASH SERIES|Exercise Level - II (Type-I) (Practice sheet - 1 (Section-A : More than one correct answer type questions))|5 VideosAROMATIC HYDROCARBONS
AAKASH SERIES|Exercise OBJECTIVE EXERCIES - 3 (RECENT AIPMT/NEET QUESTIONS)|10 VideosCHEMICAL BONDING
AAKASH SERIES|Exercise OBJECTIVE EXERCISE -3 (RECENT AIPMT/NEET QUESTIONS )|39 Videos
Similar Questions
Explore conceptually related problems
The only electron in the hydrogen atom resides under ordinary conditions on the first orbit. When energy is supplied, the electron moves to higher energy orbit depending on the amount of energy absorbed. When this electron returns to any of the lower orbits, it emits energy. Lyman series is formed when the electron returns to the lowest orbit while Balmer series is formed when the electron returns to second orbit. Similarly, Paschen, Brackett and Pfund series are formed when electron returns to the third, fourth orbits from higher energy orbits respectively (as shown in figure) Maximum number of lines produced when an electron jumps from nth level to ground level is equal to (n(n-1))/(2) . For example, in the case of n = 4, number of lines produced is 6. (4 rarr 3, 4 rarr 2, 4 rarr 1, 3 rarr 2, 3 rarr 1, 2 rarr 1) . When an electron returns from n_(2) to n_(1) state, the number of lines in the spectrum will be equal to ((n_(2) - n_(1))(n_(2)-n_(1) +1))/(2) If the electron comes back from energy level having energy E_(2) to energy level having energy E_(2) then the difference may be expressed in terms of energy of photon as E_(2) - E_(1) = Delta E, lambda = (h c)/(Delta E) . Since h and c are constant, Delta E corresponds to definite energy, thus each transition from one energy level to another will prouce a higher of definite wavelength. THis is actually observed as a line in the spectrum of hydrogen atom. Wave number of the line is given by the formula bar(v) = RZ^(2)((1)/(n_(1)^(2)) - (1)/(n_(2)^(2))) Where R is a Rydberg constant (R = 1.1 xx 10^(7)) (i) First line of a series : it is called .line of logest wavelength. or .line of shortest energy.. (ii) Series limit of last of a series : It is the line of shortest wavelength or line of highest energy. The difference in the wavelength of the 2^(nd) line of Lyman series and last line of Bracket series in a hydrogen sample is
The only electron in the hydrogen atom resides under ordinary conditions on the first orbit. When energy is supplied, the electron moves to higher energy orbit depending on the amount of energy absorbed. When this electron returns to any of the lower orbits, it emits energy. Lyman series is formed when the electron returns to the lowest orbit while Balmer series is formed when the electron returns to second orbit. Similarly, Paschen, Brackett and Pfund series are formed when electron returns to the third, fourth orbits from higher energy orbits respectively (as shown in figure) Maximum number of lines produced when an electron jumps from nth level to ground level is equal to (n(n-1))/(2) . For example, in the case of n = 4, number of lines produced is 6. (4 rarr 3, 4 rarr 2, 4 rarr 1, 3 rarr 2, 3 rarr 1, 2 rarr 1) . When an electron returns from n_(2) to n_(1) state, the number of lines in the spectrum will be equal to ((n_(2) - n_(1))(n_(2)-n_(1) +1))/(2) If the electron comes back from energy level having energy E_(2) to energy level having energy E_(2) then the difference may be expressed in terms of energy of photon as E_(2) - E_(1) = Delta E, lambda = (h c)/(Delta E) . Since h and c are constant, Delta E corresponds to definite energy, thus each transition from one energy level to another will prouce a higher of definite wavelength. THis is actually observed as a line in the spectrum of hydrogen atom. Wave number of the line is given by the formula bar(v) = RZ^(2)((1)/(n_(1)^(2)) - (1)/(n_(2)^(2))) Where R is a Rydberg constant (R = 1.1 xx 10^(7)) (i) First line of a series : it is called .line of logest wavelength. or .line of shortest energy.. (ii) Series limit of last of a series : It is the line of shortest wavelength or line of highest energy. In a single isolated atom in electron makes transition from 5^(th) excited state to 2^(nd) state the maximum number of different types of photons observed as
The only electron in the hydrogen atom resides under ordinary conditions on the first orbit. When energy is supplied, the electron moves to higher energy orbit depending on the amount of energy absorbed. When this electron returns to any of the lower orbits, it emits energy. Lyman series is formed when the electron returns to the lowest orbit while Balmer series is formed when the electron returns to second orbit. Similarly, Paschen, Brackett and Pfund series are formed when electron returns to the third, fourth orbits from higher energy orbits respectively (as shown in figure) Maximum number of lines produced when an electron jumps from nth level to ground level is equal to (n(n-1))/(2) . For example, in the case of n = 4, number of lines produced is 6. (4 rarr 3, 4 rarr 2, 4 rarr 1, 3 rarr 2, 3 rarr 1, 2 rarr 1) . When an electron returns from n_(2) to n_(1) state, the number of lines in the spectrum will be equal to ((n_(2) - n_(1))(n_(2)-n_(1) +1))/(2) If the electron comes back from energy level having energy E_(2) to energy level having energy E_(2) then the difference may be expressed in terms of energy of photon as E_(2) - E_(1) = Delta E, lambda = (h c)/(Delta E) . Since h and c are constant, Delta E corresponds to definite energy, thus each transition from one energy level to another will prouce a higher of definite wavelength. THis is actually observed as a line in the spectrum of hydrogen atom. Wave number of the line is given by the formula bar(v) = RZ^(2)((1)/(n_(1)^(2)) - (1)/(n_(2)^(2))) Where R is a Rydberg constant (R = 1.1 xx 10^(7)) (i) First line of a series : it is called .line of logest wavelength. or .line of shortest energy.. (ii) Series limit of last of a series : It is the line of shortest wavelength or line of highest energy. Let v_(1) be the frequency of the series limit of the Lyman series, v_(2) be the frequency of the first line of the Lyman series, and v_(3) be the frequency of the series limit of the Balmer series
Brakett series is produced when the electrons from outer orbits jumps to
The only e^- in the H-atom resides under ordinary conditions on the first orbit when energy is supplied, the e^- moves to higher energy shells depending upon the amount of energy absorbed. When an e emits energy i.e., the e^- returns to the lowest energy state, from this Lyman, Balmer, Paschen, Bracket, Pfund series are there, so different spectral lines in the spectra of atoms correspond to different transitions of e^- s from higher to lower energy levels: The ratio of number of spectral lines obtained when an e^- s jumps from 7^(th) to ground to 6^(th) to 3^(rd)
The only e^- in the H-atom resides under ordinary conditions on the first orbit when energy is supplied, the e^- moves to higher energy shells depending upon the amount of energy absorbed. When an e emits energy i.e., the e^- returns to the lowest energy state, from this Lyman, Balmer, Paschen, Bracket, Pfund series are there, so different spectral lines in the spectra of atoms correspond to different transitions of e^- s from higher to lower energy levels: In an hydrogen atom which of the following transition should be associated with highest absorption of energy
Brackett series is produced when the electrons from outer orbits jump to
When electrons jump from higher energy orbits to third energy orbit spectral lines emitted will belong to
AAKASH SERIES-ATOMIC STRUCTURE-Level - II (Type-I) (Practice sheet - 1 (Section - B : Linked comprehension Type questions))
- The only electron in the hydrogen atom resides under ordinary conditio...
Text Solution
|
- The only electron in the hydrogen atom resides under ordinary conditio...
Text Solution
|
- The only electron in the hydrogen atom resides under ordinary conditio...
Text Solution
|
- The only electron in the hydrogen atom resides under ordinary conditio...
Text Solution
|
- The only electron in the hydrogen atom resides under ordinary conditio...
Text Solution
|