Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
SL ARORA-Kinetic Theory of gases-Example
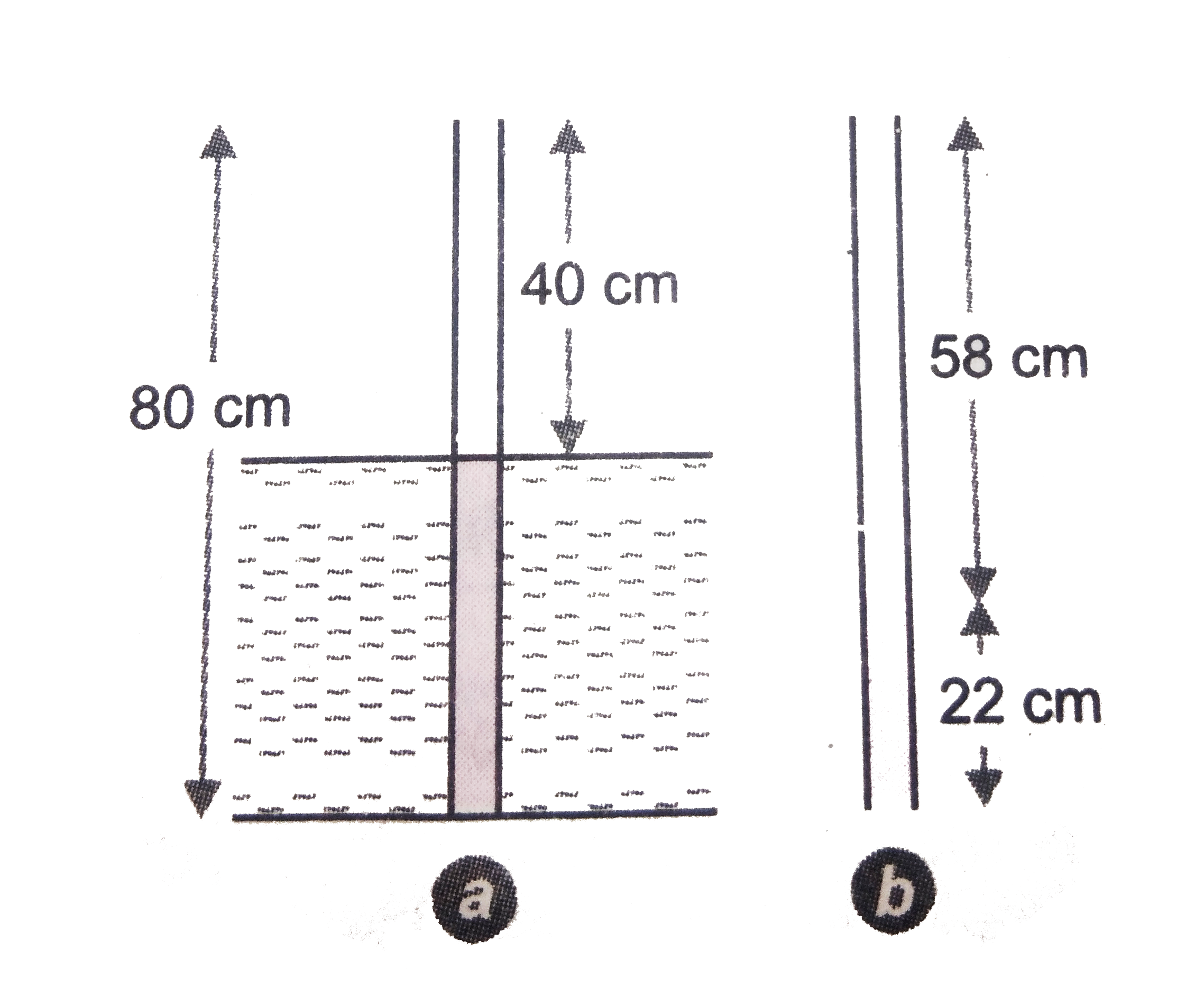
- A narrow uniform glass tube 80 cm long and open at both ends is half i...
Text Solution
|
- A gas at 27^@ C in a cylinder has a volume of 4 litre and pressure 100...
Text Solution
|
- A gas at 27^@ C in a cylinder has a volume of 4 litre and pressure 100...
Text Solution
|
- As an air bubble rises from the bottom of a lake to the surface, its v...
Text Solution
|
- Using the ideal gas equation, determine the value of gas constant R. G...
Text Solution
|
- A balloon partially filled with helium has a volume of 30 m^3, at the ...
Text Solution
|
- A vessel contains two non-reactive gases neon (monoatomic) and oxygen ...
Text Solution
|
- A vessel contains two non-reactive gases neon (monoatomic) and oxygen ...
Text Solution
|
- A closed container of volume 0.02m^(3) contains a mixture of neon and ...
Text Solution
|
- Calculate the r.m.s. velocity of air molecules at S.T.P. Given density...
Text Solution
|
- A vessel is filled with a gas at a pressure of 76 cm of mercury at a c...
Text Solution
|
- Calculate the kinetic energy of one mole of argon at 127^(@)C. Given,B...
Text Solution
|
- Calculate the KE per molecule and also rms velocity of a gas at 127^(@...
Text Solution
|
- Calculate the number of molecule in 2 xx 10^(-6)m^(3) of a perfect gas...
Text Solution
|
- Calculate the root mean square speed of one gram molecule of hydrogen ...
Text Solution
|
- (a) Calculate (i) root-mean-square speed and (ii) the mean energy of 1...
Text Solution
|
- Calculate Boltzmann's constent.
Text Solution
|
- At what temperature will the average velocity of oxygen molecules be s...
Text Solution
|
- A vessel A contains hydrogen and another vessel B whose volume is twic...
Text Solution
|
- A vessel A contains hydrogen and another vessel B whose volume is twic...
Text Solution
|