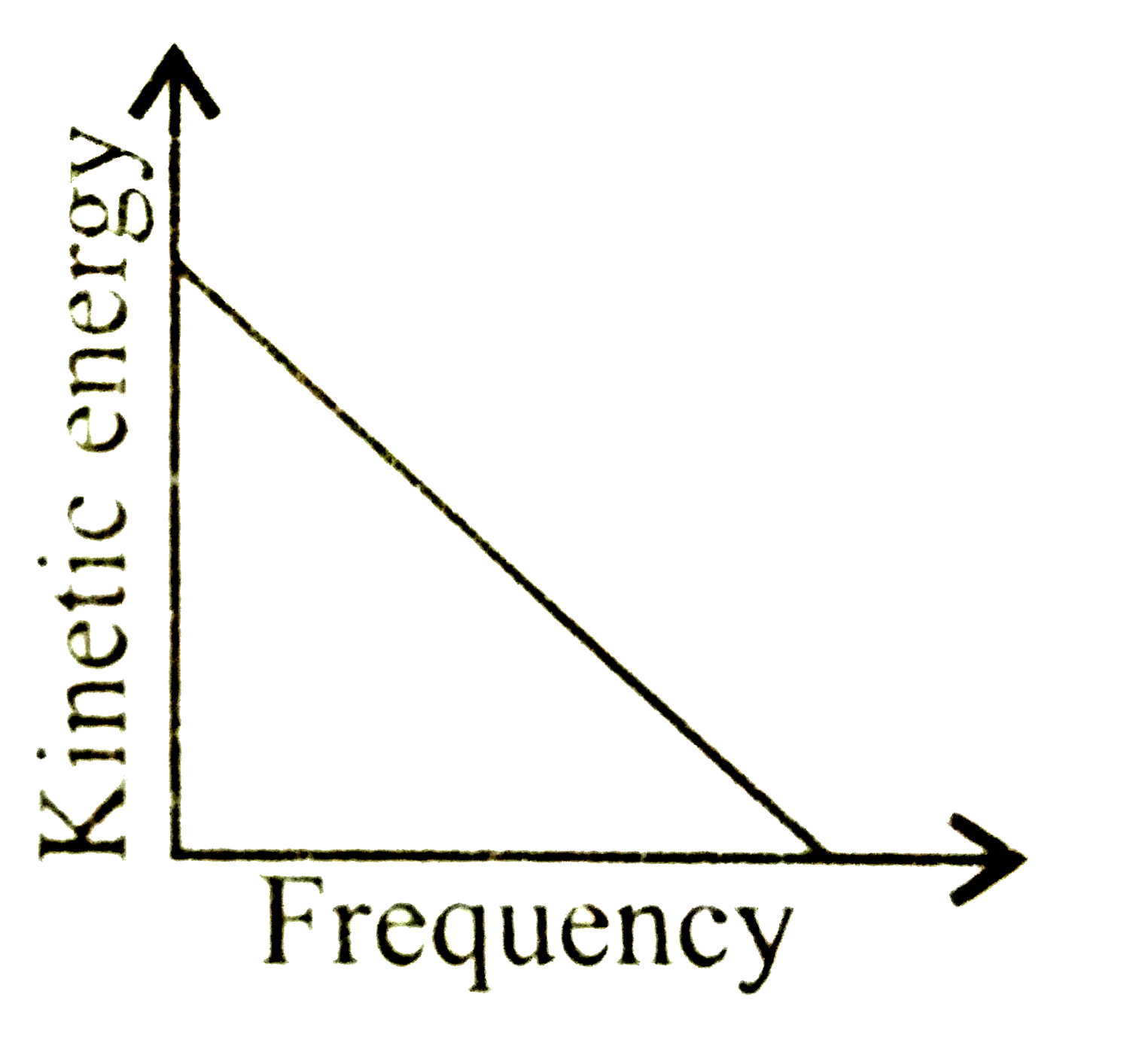
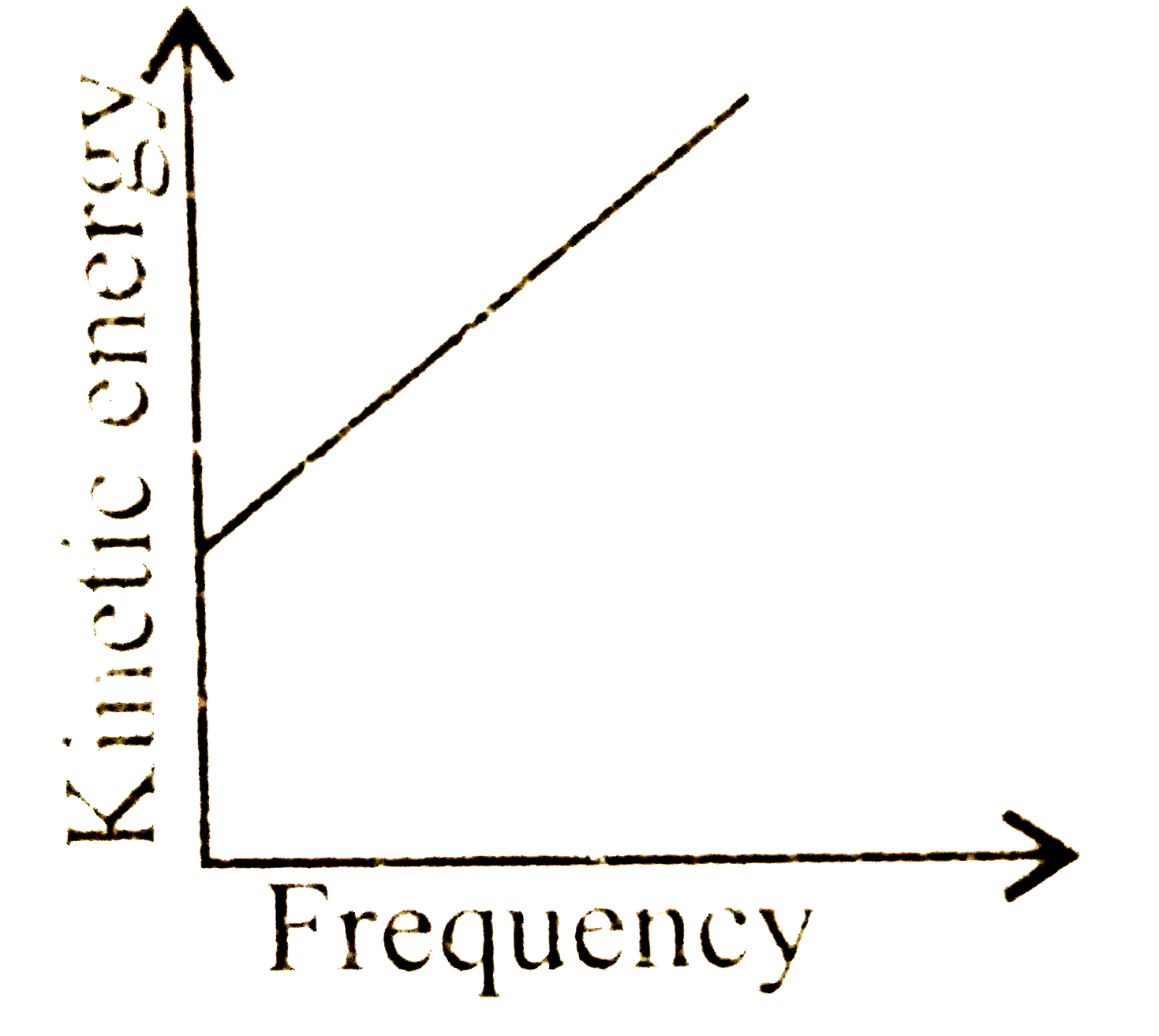
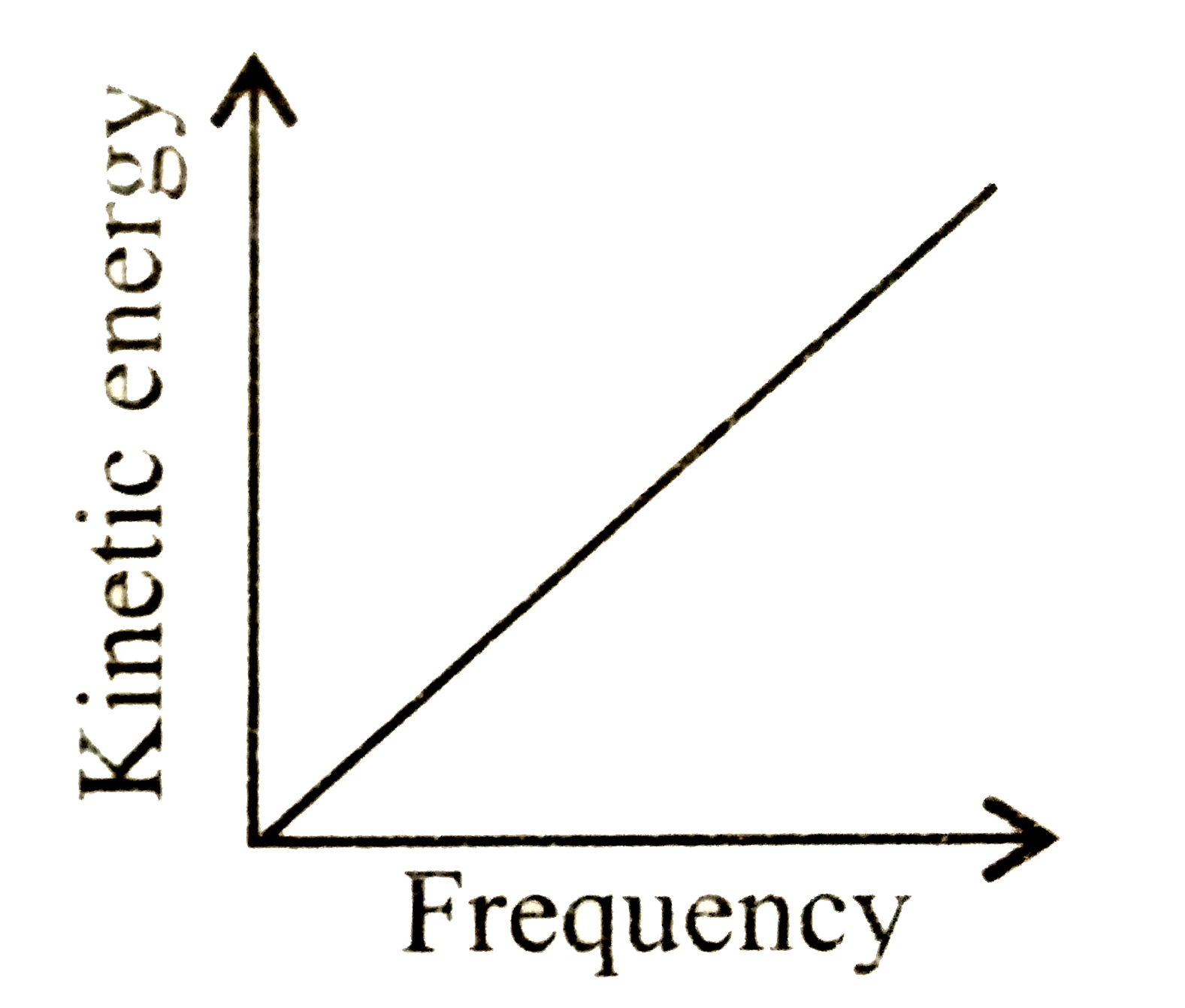
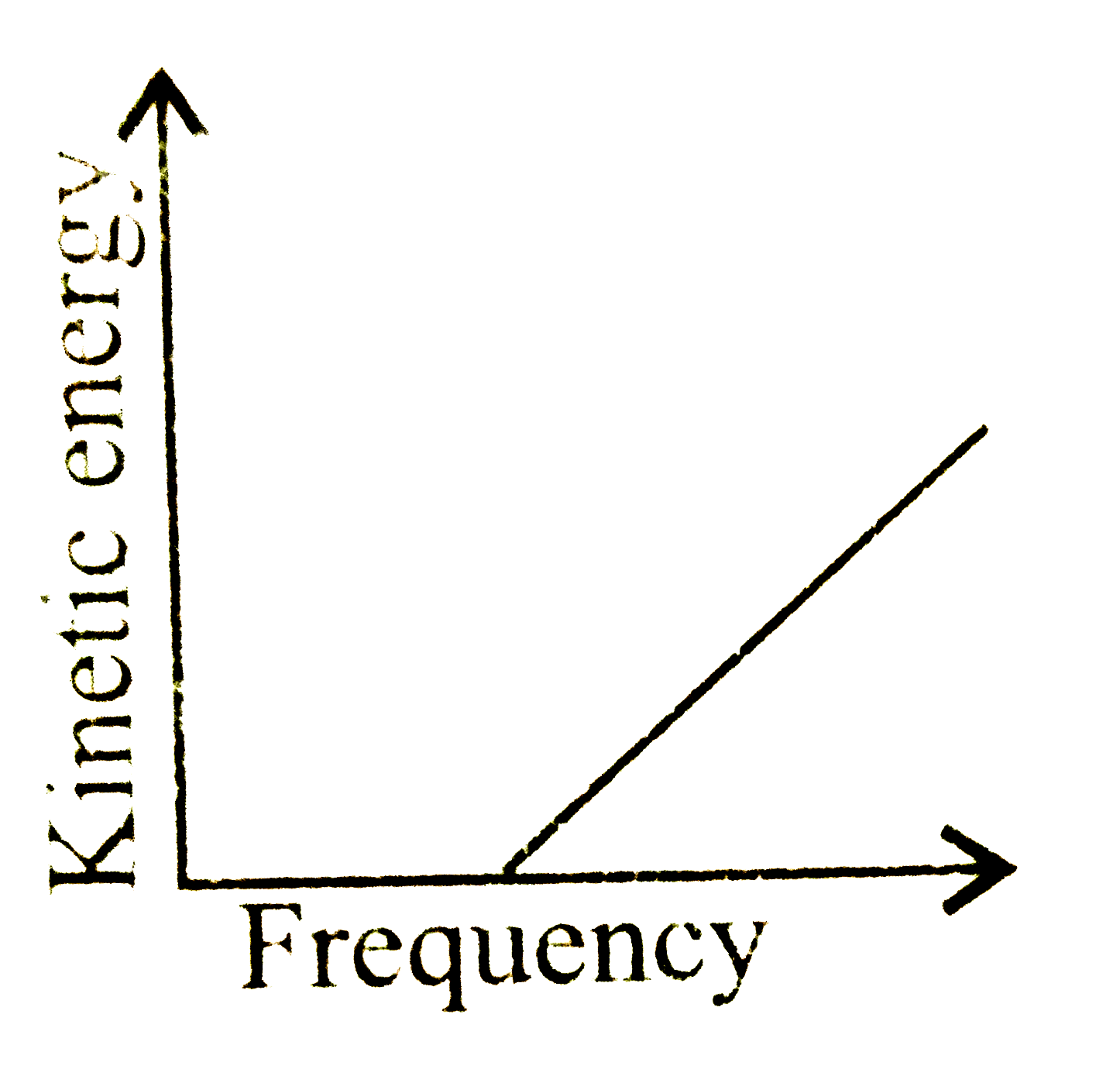
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
DUAL NATURE OF RADIATION AND MATTER
NCERT FINGERTIPS ENGLISH|Exercise PARTICLE NATURE OF LIGHT : THE PHOTON|19 VideosDUAL NATURE OF RADIATION AND MATTER
NCERT FINGERTIPS ENGLISH|Exercise WAVE NATURE OF MATTER|37 VideosDUAL NATURE OF RADIATION AND MATTER
NCERT FINGERTIPS ENGLISH|Exercise EXPERIMENTAL STUDY OF PHOTOELECTRIC EFFECT|11 VideosCURRENT ELECTRICITY
NCERT FINGERTIPS ENGLISH|Exercise Assertion And Reason|15 VideosELECTRIC CHARGES AND FIELDS
NCERT FINGERTIPS ENGLISH|Exercise Assertion And Reason|15 Videos
Similar Questions
Explore conceptually related problems
NCERT FINGERTIPS ENGLISH-DUAL NATURE OF RADIATION AND MATTER -EINSTEIN S PHOTOELECTRIC EQUATION : ENERGY QUANTUM OF RADIATION
- According to Einstein's photoelectric equation , the graph between the...
Text Solution
|
- According to Einstein's photoelectric equation, the plot of the maximu...
Text Solution
|
- In a photoelectric experiment, the graph of frequency v of incident li...
Text Solution
|
- A student performs an experiment on photoelectric effect using two mat...
Text Solution
|
- Light of wavelength 0.6 mum from a sodium lamp falls on a photocell an...
Text Solution
|
- When the photons of energy hv fall on a photosensitive metallic surfac...
Text Solution
|
- The photoeletric threshold 4v is incident on the metal is v. When ligh...
Text Solution
|
- Photoelectric emission occurs only when the incident light has more th...
Text Solution
|
- The work function for Al, K and Pt is 4.28 eV, 2.30 eV and 5.65 eV res...
Text Solution
|
- The photoelectric threshold wavelength for silver is lamda(0). The ene...
Text Solution
|
- A photon of energy E ejects a photoelectron from a metel surface whose...
Text Solution
|
- Two identical photocathodes receive light of frequencies v(1) and v(2)...
Text Solution
|
- A metallic surface is irradiated by a monochromatic light of frequency...
Text Solution
|
- If K(1) and K(2) are maximum kinetic energies of photoelectrons emitte...
Text Solution
|
- The work function of cesium is 2.14 eV. The threshold freqency of caes...
Text Solution
|
- The work function of Cs is 2.14 ev find the wavelength of the incident...
Text Solution
|
- represents a graph of most energetic photoelectrons K(max)(in eV) and ...
Text Solution
|
- Light of frequency 7.21xx10^(14)Hz is incident on a metal surface. Ele...
Text Solution
|
- For a certain metal v is the five times of v(0) and the maximum veloci...
Text Solution
|
- A light of wavelength 600 nm is incident on a metal surface. When ligh...
Text Solution
|