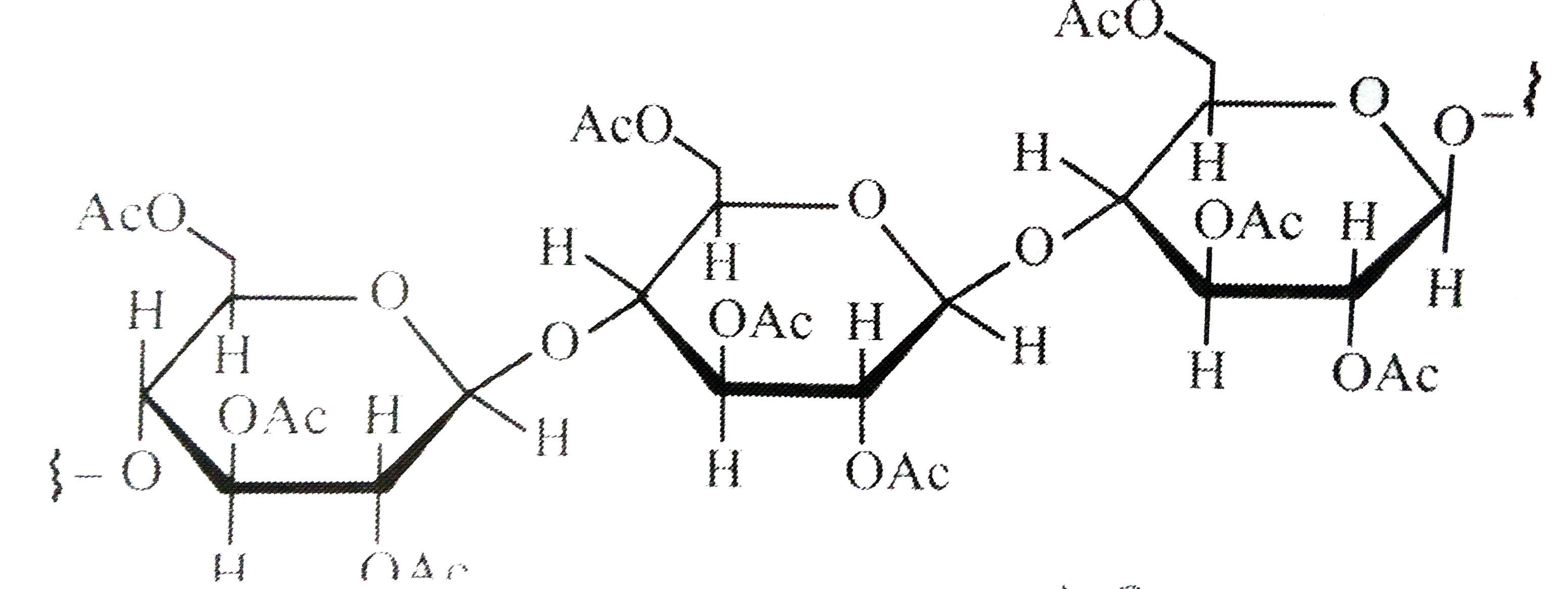
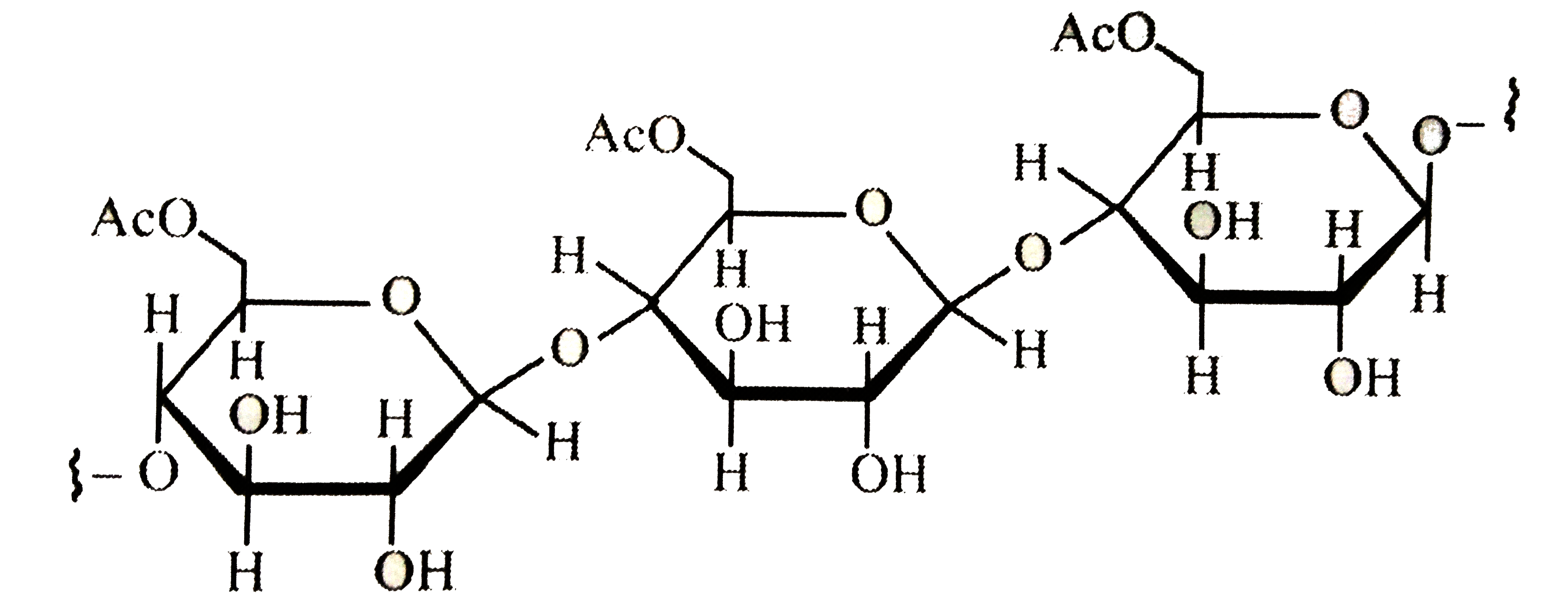
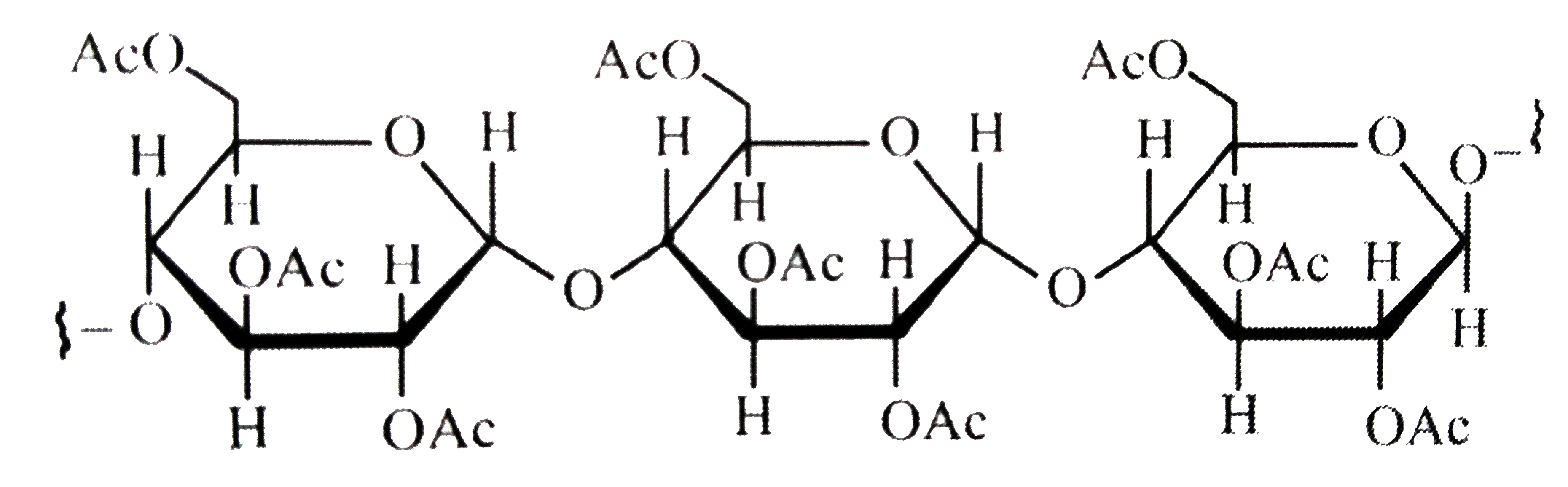
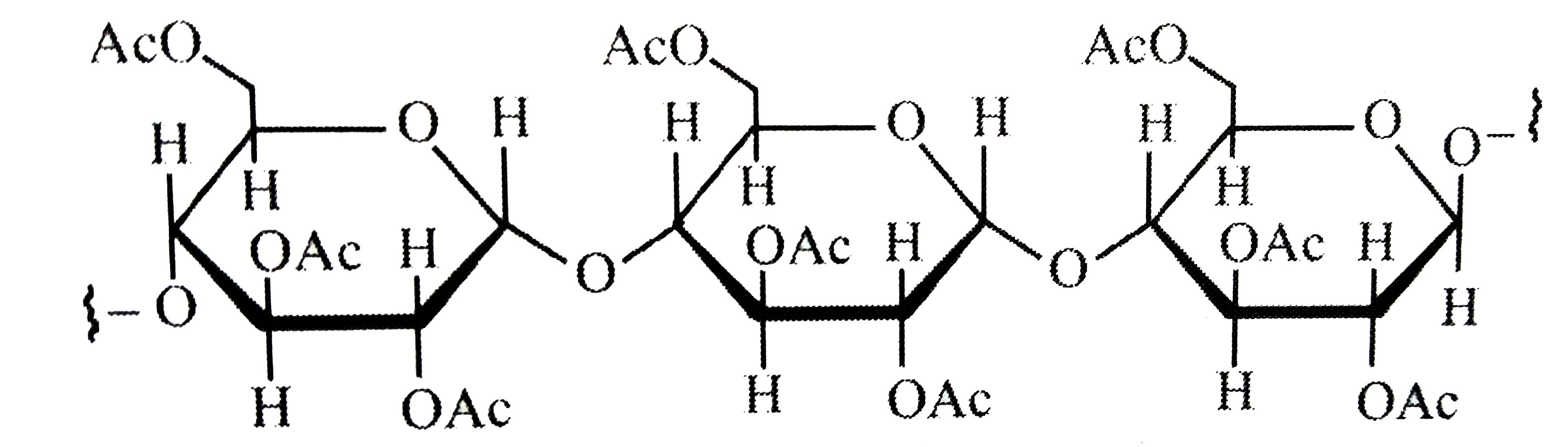
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
BIOMOLECULES
NCERT FINGERTIPS ENGLISH|Exercise EXEMPLER PROBLEMS|16 VideosBIOMOLECULES
NCERT FINGERTIPS ENGLISH|Exercise ASSERTION & REASON|15 VideosBIOMOLECULES
NCERT FINGERTIPS ENGLISH|Exercise Assertion And Reason|15 VideosAMINES
NCERT FINGERTIPS ENGLISH|Exercise ASSERTION & REASON CORNER|14 VideosCHEMICAL KINETICS
NCERT FINGERTIPS ENGLISH|Exercise Assertion And Reason|15 Videos
Similar Questions
Explore conceptually related problems
NCERT FINGERTIPS ENGLISH-BIOMOLECULES-HOTS
- Cellulose upon acetylation with excess acetic anhydride/H(2)SO(4) (cat...
Text Solution
|
- During the process of digestion, the proteins present in food material...
Text Solution
|
- Within the list shown below, the correct pair of structures of alanine...
Text Solution
|
- In aqueous solution amino acids moslty exit as
Text Solution
|
- Amino acids are least soluble
Text Solution
|