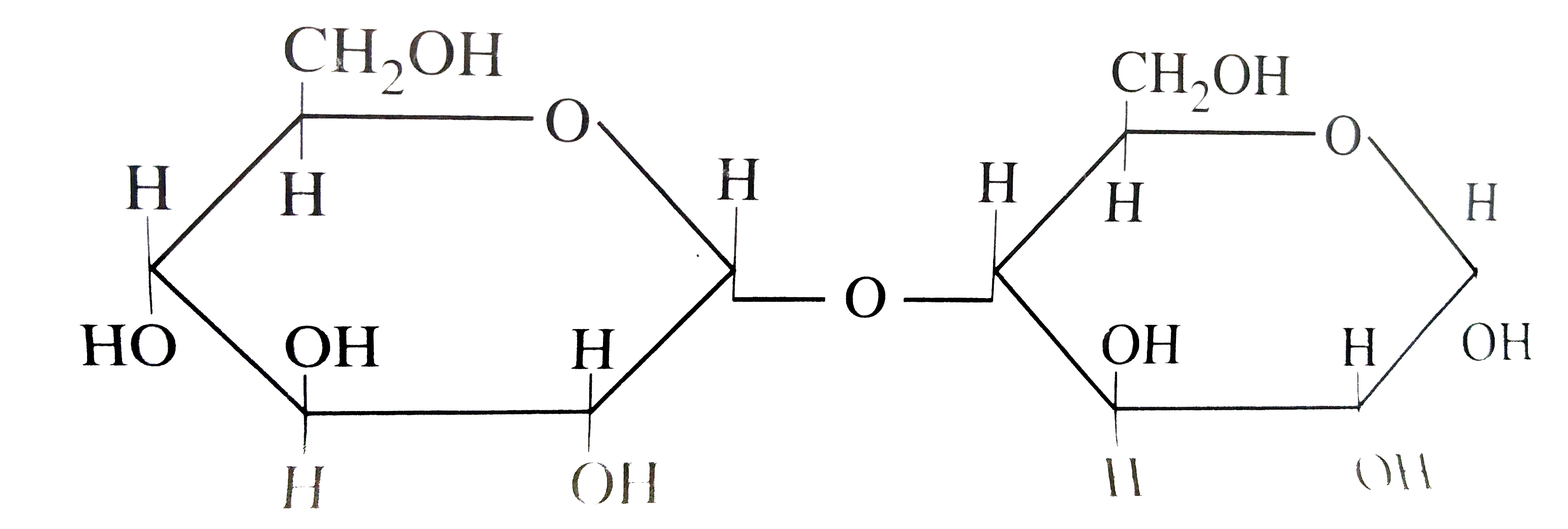
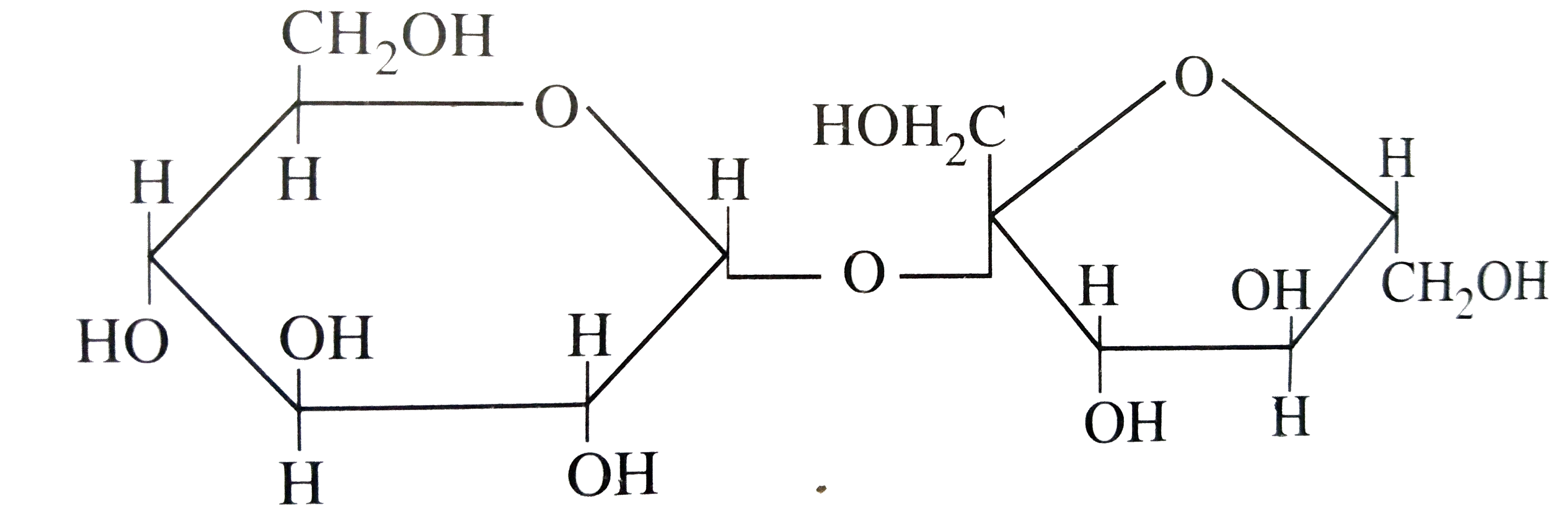
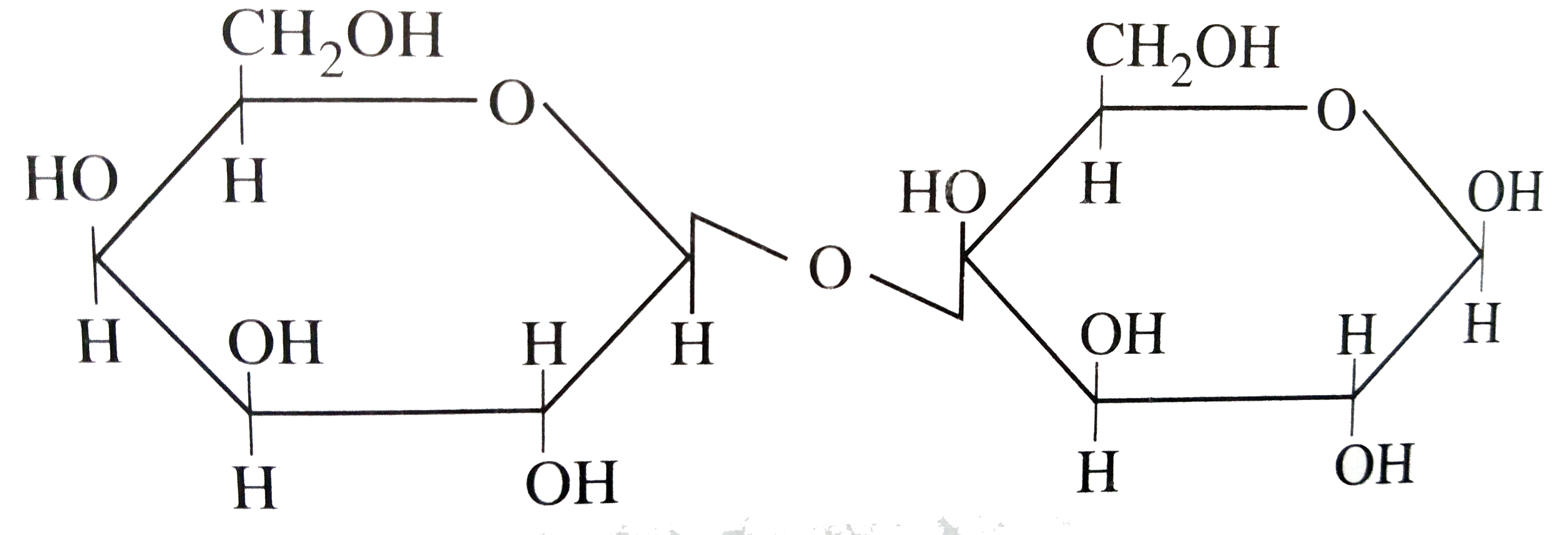
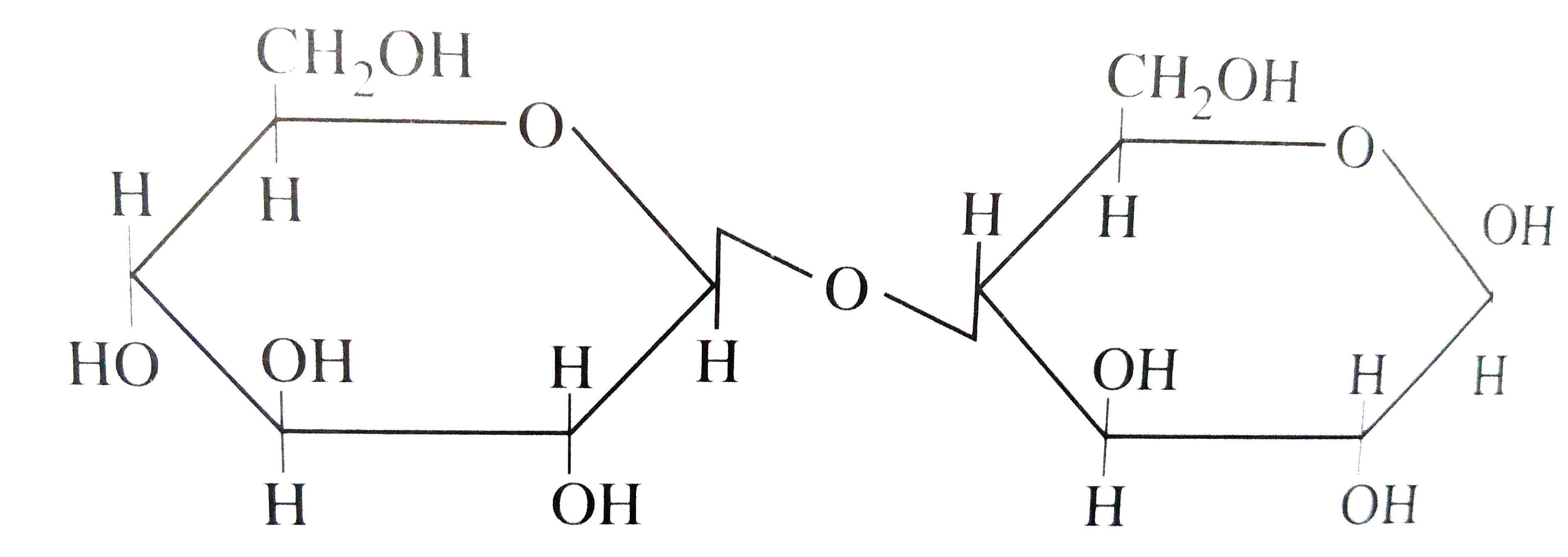
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
BIOMOLECULES
NCERT FINGERTIPS ENGLISH|Exercise ASSERTION & REASON|15 VideosBIOMOLECULES
NCERT FINGERTIPS ENGLISH|Exercise Carbohydrates|35 VideosBIOMOLECULES
NCERT FINGERTIPS ENGLISH|Exercise HOTS|5 VideosAMINES
NCERT FINGERTIPS ENGLISH|Exercise ASSERTION & REASON CORNER|14 VideosCHEMICAL KINETICS
NCERT FINGERTIPS ENGLISH|Exercise Assertion And Reason|15 Videos
Similar Questions
Explore conceptually related problems
NCERT FINGERTIPS ENGLISH-BIOMOLECULES-EXEMPLER PROBLEMS
- Glycogen is a branched chain polymer of alpha-D glucose units in which...
Text Solution
|
- Which of the following polymer is stored in the liver of animals ?
Text Solution
|
- Sucrose (cane sugar) is a disaccharide. One molecule of sucrose on hyd...
Text Solution
|
- Which of the following pairs represents anomers ?
Text Solution
|
- Proteins are found to have two different types of secondary structures...
Text Solution
|
- In disaccharides, if the reducing groups of monosaccharides, i.e., ald...
Text Solution
|
- Which of the following acids is a vitamin?
Text Solution
|
- Dinucleotide is obtained by joining two nucleotides together by phosph...
Text Solution
|
- Nucleic acids are the polymers of
Text Solution
|
- Which of the following statements is not true about glucose?
Text Solution
|
- Each polypeptide in a protein has amino acids linked with each other i...
Text Solution
|
- DNA and RNA contain four bases each. Which of the following bases in n...
Text Solution
|
- Which of the following B group vitamins can be stored in our body?
Text Solution
|
- Which of the following bases is not present in DNA
Text Solution
|
- Which of the following reactions of glucose can be explained only by i...
Text Solution
|
- Structure of a disaccharide formed by glucose and fructose is given be...
Text Solution
|