Text Solution
Verified by Experts
Topper's Solved these Questions
HALOALKANES & HALOARENES
OSWAAL PUBLICATION|Exercise Topic-1 (Some important terms and Definitions) (Long Answer Type Questons-I)|3 VideosHALOALKANES & HALOARENES
OSWAAL PUBLICATION|Exercise Topic-1 (Some important terms and Definitions) (Long Answer Type Questons-II)|1 VideosHALOALKANES & HALOARENES
OSWAAL PUBLICATION|Exercise Topic 2 (Properties of Haloarenes and Haloalkanes) (Long Answer Type Questions - II)|1 VideosGENERAL PRINCIPLES AND PROCESSES OF ISOLATION OF ELEMENTS
OSWAAL PUBLICATION|Exercise TOPIC 2 (LONG ANSWER TYPE QUESTIONS-II)|2 VideosII PUC ANNUAL EXAMINATION 2019
OSWAAL PUBLICATION|Exercise PART - D|10 Videos
Similar Questions
Explore conceptually related problems
OSWAAL PUBLICATION-HALOALKANES & HALOARENES-Topic-1 (Some important terms and Definitions) (Short Answer Type Questions)
- What is optical activity ? Which one of the following compounds shows ...
Text Solution
|
- Write structures of different dihalogen derivatives of propane.
Text Solution
|
- Write structures of the following compounds. (i) 2-chloro-3-methylpe...
Text Solution
|
- Write the isomers of the compound having formula C(4)H(9)Br.
Text Solution
|
- What are ambident nucleophiles ? Explain write an examples too.
Text Solution
|
- Give IUPAC name of the following compound. (i) CH(3)CH(CI)CH(Br)CH(3...
Text Solution
|
- Which one of the following has highest dipole moment ?
Text Solution
|
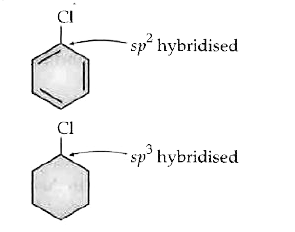
- Why the dipole moment of chlorobenzene is lower than that of cyclohexy...
Text Solution
|
- Why alkyl halides, though polar, are immiscible with water ?
Text Solution
|