Text Solution
Verified by Experts
Topper's Solved these Questions
ELECTROCHEMISTRY
KUMAR PRAKASHAN|Exercise SECTION-A (Try Yourself)|25 VideosELECTROCHEMISTRY
KUMAR PRAKASHAN|Exercise SECTION-A (Practice Questions)|3 VideosELECTROCHEMISTRY
KUMAR PRAKASHAN|Exercise SECTION-E MCQ.s Asked in GUJCET/Board Exam|55 VideosCHEMISTRY IN EVERYDAY LIFE
KUMAR PRAKASHAN|Exercise SECTION - E (MCQs ASKED IN GUJCET/BOARD EXAM)|37 VideosGENERAL PRINCIPLES AND PROCESSES OF ISOLATION OF ELEMENTS
KUMAR PRAKASHAN|Exercise SECTION - E (MCQs ASKED IN GUJET/BOARD EXAMS)|46 Videos
Similar Questions
Explore conceptually related problems
KUMAR PRAKASHAN-ELECTROCHEMISTRY-SECTION-A
- Give cel reaction, expression and formula for copper - Silver galvanic...
Text Solution
|
- What is to be done to determine cell potential ?
Text Solution
|
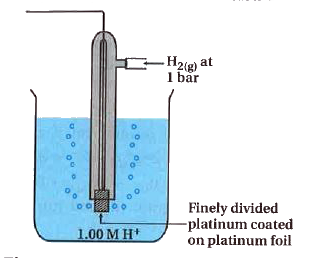
- Write a note on standard hydrogen electrode.
Text Solution
|
- Discuss the method to determine cell potential of any cell when standa...
Text Solution
|
- Discuss the method to determine cell potential of any cell when standa...
Text Solution
|
- Calculate the Zn-Cu cell (Daniell) potential.
Text Solution
|
- What is electrode and half-cell ? Explain symbolic representationby su...
Text Solution
|
- Explain symbolic representation of galvanic cell (electrochemical cell...
Text Solution
|
- Give the type and examples of electrodes.
Text Solution
|
- What is the standard electrode (Half-cell) potential ? Give its uses.
Text Solution
|
- Give Nernst equation for following reaction M((aq))^(n+)+n e^(-)to M...
Text Solution
|
- Derive Nernst equation for calculating E(cell) of Nernst equation and ...
Text Solution
|
- Derive Nernst equation for the following galvanic cell. Ni((S))|Ni((...
Text Solution
|
- For general redox reaction: aA+bB overset(n e^(-)) to cC=dD. Derive ...
Text Solution
|
- Explain equilibrium state in Daniell cell and derive its equilibrium c...
Text Solution
|
- Derive an equation for equilibrium constant K(c) of any galvanic cell ...
Text Solution
|
- Write a note on relation between Gibbs free energy and cell potential ...
Text Solution
|
- What is an electric resistance ? Give note on it.
Text Solution
|
- Define resistance or specific resistance and write a note on it.
Text Solution
|
- What is conductivity ? Give note on it.
Text Solution
|