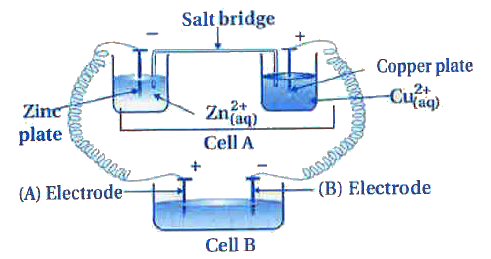
Text Solution
Verified by Experts
Topper's Solved these Questions
ELECTROCHEMISTRY
KUMAR PRAKASHAN|Exercise SECTION-E Darpan.s Exam Oriented MCQs|49 VideosELECTROCHEMISTRY
KUMAR PRAKASHAN|Exercise SECTION-E MCQ.s asked in Competitive Exam|24 VideosELECTROCHEMISTRY
KUMAR PRAKASHAN|Exercise SECTION-D NCERT Exemplar Solution (Assertion and Reason Type)|10 VideosCHEMISTRY IN EVERYDAY LIFE
KUMAR PRAKASHAN|Exercise SECTION - E (MCQs ASKED IN GUJCET/BOARD EXAM)|37 VideosGENERAL PRINCIPLES AND PROCESSES OF ISOLATION OF ELEMENTS
KUMAR PRAKASHAN|Exercise SECTION - E (MCQs ASKED IN GUJET/BOARD EXAMS)|46 Videos
Similar Questions
Explore conceptually related problems