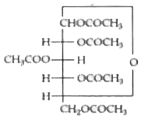
Text Solution
Verified by Experts
Topper's Solved these Questions
BIOMOLECULES
OSWAAL PUBLICATION|Exercise TOPIC - I( LONG ANSWER TYPE QUESTIONS)|1 VideosBIOMOLECULES
OSWAAL PUBLICATION|Exercise TOPIC -II (VERY SHORT ANSWER TYPE QUESTIONS)|5 VideosBIOMOLECULES
OSWAAL PUBLICATION|Exercise TOPIC -II (LONG ANSWER TYPE QUESTIONS)|2 VideosALDEHYDES, KETONES AND CARBOXYLIC ACIDS
OSWAAL PUBLICATION|Exercise TOPIC -3 METHODS OF PREPARATION OF CARBOXYLIC ACID, PROPERTIES AND USES (LONG ANSWER TYPE QUESTIONS II)|7 VideosCHEMICAL KINETICS
OSWAAL PUBLICATION|Exercise Topic - 2 (ORDER OF A REACTION , INTEGRATED RATE EQUATIONS AND HALF LIFE OF A REACTION )Long Answer Type Questions - II)|4 Videos
Similar Questions
Explore conceptually related problems
OSWAAL PUBLICATION-BIOMOLECULES-TOPIC - I( SHORT ANSWER TYPE QUESTIONS)
- Give a reaction to show glucose molecule contains . (i) Five hydr...
Text Solution
|
- Explain what is the meant by the following : (i) Peptide linkage...
Text Solution
|
- Define the following terms : (i) Glycosidic linkage . (ii) Invert...
Text Solution
|
- Explain why : (i) Glucose is soluble in water but cyclohexane is n...
Text Solution
|
- Explain the following terms : (i) Inverts sugar . (ii) Polypep...
Text Solution
|
- Write any two reactions of glucose which cannot be explained by the ...
Text Solution
|
- Enumerate the reaction and facts of D - glucose which cannot be expl...
Text Solution
|