Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
JEE MAINS PREVIOUS YEAR-JEE MAIN 2021-Chemistry (Section B)
- A xenon compound 'A' upon partial hydrolysis gives XeO2F2. The number ...
Text Solution
|
- A solute A dimerizes in water. The boiling point of a 2 molal solution...
Text Solution
|
- A reaction has a half life of 1min. The time required for 99.9% comple...
Text Solution
|
- The solubility of CdSO4 in water is 8.0 xx 10^(-4) mol L^(-1). Its sol...
Text Solution
|
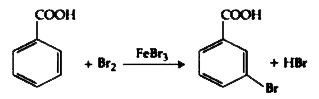
- Consider the above reaction where 6.1 g of Benzoic acid is used to get...
Text Solution
|
- The number of species below that have two lone pairs of electrons in t...
Text Solution
|
- The gas phase raction 2A(g) Leftrightarrow A(2)(g) at 400 K has tr...
Text Solution
|
- The molar conductivities at infinite dilution of barium chloride, sulp...
Text Solution
|
- 10.0mL of Na2CO3 solution is titrated against 0.2M HCl solution. The f...
Text Solution
|
- In Tollen,s test for aldehyde, the overall number of electron(s) trans...
Text Solution
|