Text Solution
Verified by Experts
Topper's Solved these Questions
SOLVED PAPER II PUC MARCH-2016
OSWAAL PUBLICATION|Exercise PART-C |11 VideosSOLVED PAPER II PUC MARCH-2016
OSWAAL PUBLICATION|Exercise PART-D |24 VideosSOLVED PAPER II PUC MARCH-2016
OSWAAL PUBLICATION|Exercise PART-D |24 VideosSOLVED PAPER II PUC JULY - 2016
OSWAAL PUBLICATION|Exercise PART -E|19 VideosSOLVED PAPER II PUC TOPPER'S ANSWER MARCH-2015
OSWAAL PUBLICATION|Exercise PART -D|30 Videos
Similar Questions
Explore conceptually related problems
OSWAAL PUBLICATION-SOLVED PAPER II PUC MARCH-2016 -PART-B
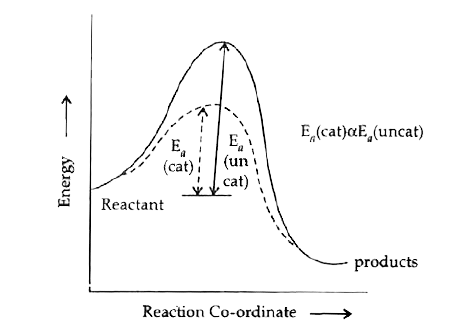
- Explain the effect of catalyst on the activation energy of the reactio...
Text Solution
|
- What is Schottkyy defect? What is the effect on the density of solids?
Text Solution
|
- Calculate the overset(@)Lambda(m) for MgCl(2). The limiting molar cond...
Text Solution
|
- Explain Clemmensen reduction with an example.
Text Solution
|
- What are analgesics ? Give one example for non-narcotic analgesic.
Text Solution
|
- Give reasons: Actinoids show variable oxidation states.
Text Solution
|
- Give reason: Why Lanthanoids are less reactive than actinoids.
Text Solution
|
- What are anionic detergents? Give an example.
Text Solution
|
- What is the action of bromine in ethanoic acid on anisole?
Text Solution
|