Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ICSE-SAMPLE PAPER 2015-PART-II SECTION-C QUESTION 10.
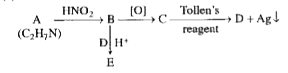
- An organic compound A with molecular formula C2 H7 N on reaction wit...
Text Solution
|
- Give balanced equations for the following reactions : How will you c...
Text Solution
|
- What is the effect of denaturation on the structure of proteins?
Text Solution
|
- Name the nitrogen base residues present in DNA ?
Text Solution
|
- Give balanced equations for the following reactions : Aniline is t...
Text Solution
|
- Give balanced equation for the following reaction : Acetyl chloride...
Text Solution
|
- Give balanced equation for the reaction: Formaldehyde is treated wit...
Text Solution
|