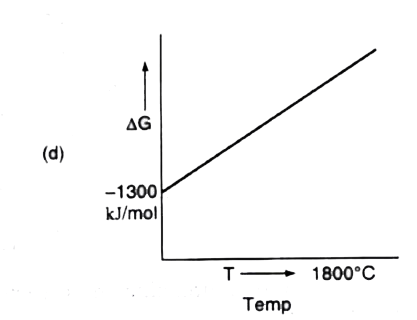
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
METALLURGY
VK JAISWAL ENGLISH|Exercise Level 3 (Passive 2)|6 VideosMETALLURGY
VK JAISWAL ENGLISH|Exercise Level 3 (Passive 3)|6 VideosMETALLURGY
VK JAISWAL ENGLISH|Exercise Level 2|86 VideosENVIRONMENTAL CHEMISTRY
VK JAISWAL ENGLISH|Exercise ASSERTION-REASON TYPE QUESTIONS|14 Videosp-BLOCK ELEMENTS
VK JAISWAL ENGLISH|Exercise SUBJECTIVE PROBLEMS|35 Videos
VK JAISWAL ENGLISH-METALLURGY-Level 3 (Passive 1)
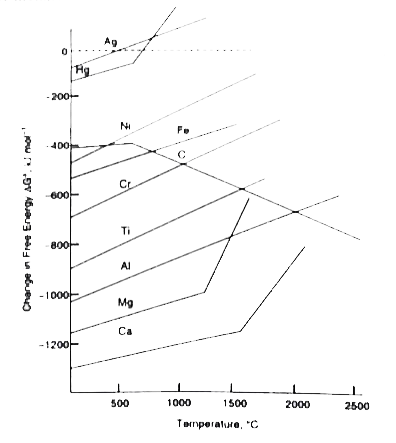
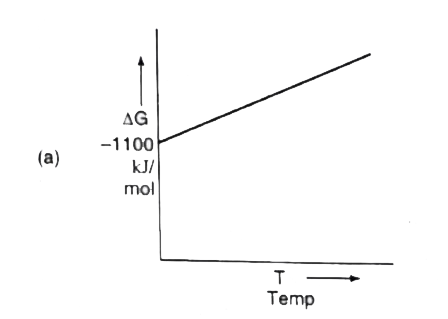
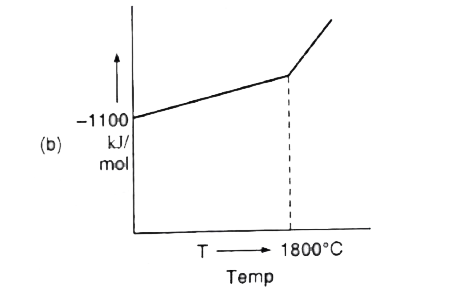
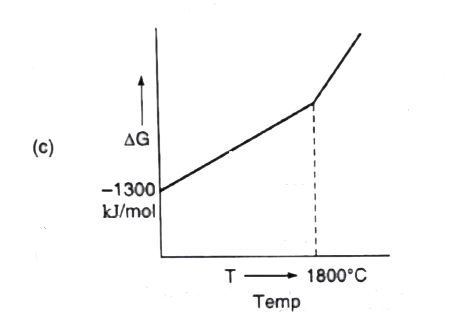
- For a sponaneous reaction, the free energy change must be negative, De...
Text Solution
|
- For a sponaneous reaction, the free energy change must be negative, De...
Text Solution
|
- For a sponaneous reaction, the free energy change must be negative, De...
Text Solution
|
- For a sponaneous reaction, the free energy change must be negative, De...
Text Solution
|
- For a sponaneous reaction, the free energy change must be negative, De...
Text Solution
|
- For a sponaneous reaction, the free energy change must be negative, De...
Text Solution
|
- For a sponaneous reaction, the free energy change must be negative, De...
Text Solution
|
- For a sponaneous reaction, the free energy change must be negative, De...
Text Solution
|